NADPH oxidases: a perspective on reactive oxygen species production in tumor biology
- PMID: 24156355
- PMCID: PMC4026372
- DOI: 10.1089/ars.2013.5603
NADPH oxidases: a perspective on reactive oxygen species production in tumor biology
Abstract
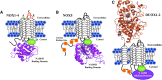
Significance: Reactive oxygen species (ROS) promote genomic instability, altered signal transduction, and an environment that can sustain tumor formation and growth. The NOX family of NADPH oxidases, membrane-bound epithelial superoxide and hydrogen peroxide producers, plays a critical role in the maintenance of immune function, cell growth, and apoptosis. The impact of NOX enzymes in carcinogenesis is currently being defined and may directly link chronic inflammation and NOX ROS-mediated tumor formation.
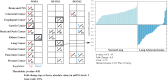
Recent advances: Increased interest in the function of NOX enzymes in tumor biology has spurred a surge of investigative effort to understand the variability of NOX expression levels in tumors and the effect of NOX activity on tumor cell proliferation. These initial efforts have demonstrated a wide variance in NOX distribution and expression levels across numerous cancers as well as in common tumor cell lines, suggesting that much remains to be discovered about the unique role of NOX-related ROS production within each system. Progression from in vitro cell line studies toward in vivo tumor tissue screening and xenograft models has begun to provide evidence supporting the importance of NOX expression in carcinogenesis.
Critical issues: A lack of universally available, isoform-specific antibodies and animal tumor models of inducible knockout or over-expression of NOX isoforms has hindered progress toward the completion of in vivo studies.
Future directions: In vivo validation experiments and the use of large, existing gene expression data sets should help define the best model systems for studying the NOX homologues in the context of cancer.
Figures


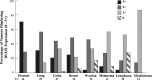

Similar articles
-
Methods for Detection of NOX-Derived Superoxide Radical Anion and Hydrogen Peroxide in Cells.Methods Mol Biol. 2019;1982:233-241. doi: 10.1007/978-1-4939-9424-3_13. Methods Mol Biol. 2019. PMID: 31172475
-
NADPH Oxidases and Measurement of Reactive Oxygen Species.Methods Mol Biol. 2017;1527:219-232. doi: 10.1007/978-1-4939-6625-7_18. Methods Mol Biol. 2017. PMID: 28116720
-
Inhibiting the Activity of NADPH Oxidase in Cancer.Antioxid Redox Signal. 2020 Aug 20;33(6):435-454. doi: 10.1089/ars.2020.8046. Epub 2020 Apr 17. Antioxid Redox Signal. 2020. PMID: 32008376 Free PMC article.
-
MicroRNA Targeting Nicotinamide Adenine Dinucleotide Phosphate Oxidases in Cancer.Antioxid Redox Signal. 2020 Feb 10;32(5):267-284. doi: 10.1089/ars.2019.7918. Epub 2019 Nov 21. Antioxid Redox Signal. 2020. PMID: 31656079 Review.
-
Detection of superoxide anion and hydrogen peroxide production by cellular NADPH oxidases.Biochim Biophys Acta. 2014 Feb;1840(2):757-67. doi: 10.1016/j.bbagen.2013.04.040. Epub 2013 May 7. Biochim Biophys Acta. 2014. PMID: 23660153 Free PMC article. Review.
Cited by
-
Protective effects of valsartan administration on doxorubicin‑induced myocardial injury in rats and the role of oxidative stress and NOX2/NOX4 signaling.Mol Med Rep. 2020 Nov;22(5):4151-4162. doi: 10.3892/mmr.2020.11521. Epub 2020 Sep 17. Mol Med Rep. 2020. PMID: 33000246 Free PMC article.
-
The Microenvironment Is a Critical Regulator of Muscle Stem Cell Activation and Proliferation.Front Cell Dev Biol. 2019 Oct 29;7:254. doi: 10.3389/fcell.2019.00254. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31737625 Free PMC article. Review.
-
The roles of reactive oxygen species and antioxidants in cryopreservation.Biosci Rep. 2019 Aug 29;39(8):BSR20191601. doi: 10.1042/BSR20191601. Print 2019 Aug 30. Biosci Rep. 2019. PMID: 31371631 Free PMC article. Review.
-
The interplay of reactive oxygen species and the epidermal growth factor receptor in tumor progression and drug resistance.J Exp Clin Cancer Res. 2018 Mar 16;37(1):61. doi: 10.1186/s13046-018-0728-0. J Exp Clin Cancer Res. 2018. PMID: 29548337 Free PMC article. Review.
-
Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling.J Cell Biol. 2018 Jun 4;217(6):1915-1928. doi: 10.1083/jcb.201708007. Epub 2018 Apr 18. J Cell Biol. 2018. PMID: 29669742 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

