Reactivation of latent HIV-1 in central memory CD4⁺ T cells through TLR-1/2 stimulation
- PMID: 24156240
- PMCID: PMC3826617
- DOI: 10.1186/1742-4690-10-119
Reactivation of latent HIV-1 in central memory CD4⁺ T cells through TLR-1/2 stimulation
Abstract
Background: Toll-like receptors (TLRs) are crucial for recognition of pathogen-associated molecular patterns by cells of the innate immune system. TLRs are present and functional in CD4⁺ T cells. Memory CD4⁺ T cells, predominantly central memory cells (TCM), constitute the main reservoir of latent HIV-1. However, how TLR ligands affect the quiescence of latent HIV within central memory CD4⁺ T cells has not been studied.
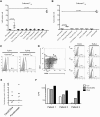
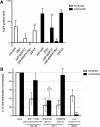
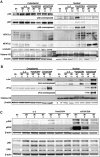
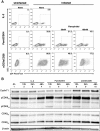
Results: We evaluated the ability of a broad panel of TLR agonists to reactivate latent HIV-1. The TLR-1/2 agonist Pam3CSK4 leads to viral reactivation of quiescent HIV in a model of latency based on cultured TCM and in resting CD4⁺ T cells isolated from aviremic patients. In addition, we investigated the signaling pathway associated with Pam3CSK4 involved in HIV-1 reactivation. We show that the transcription factors NFκB, NFAT and AP-1 cooperate to induce viral reactivation downstream of TLR-1/2 stimulation. Furthermore, increasing levels of cyclin T1 is not required for TLR-mediated viral reactivation, but induction of viral expression requires activated pTEFb. Finally, Pam3CSK4 reactivates latent HIV-1 in the absence of T cell activation or proliferation, in contrast to antigen stimulation.
Conclusions: Our findings suggest that the signaling through TLR-1/2 pathway via Pam3CSK4 or other reagents should be explored as an anti-latency strategy either alone or in combination with other anti-latency drugs.
Figures


Similar articles
-
Toll-like receptor 3 activation selectively reverses HIV latency in microglial cells.Retrovirology. 2017 Feb 6;14(1):9. doi: 10.1186/s12977-017-0335-8. Retrovirology. 2017. PMID: 28166799 Free PMC article.
-
Dual TLR2 and TLR7 agonists as HIV latency-reversing agents.JCI Insight. 2018 Oct 4;3(19):e122673. doi: 10.1172/jci.insight.122673. JCI Insight. 2018. PMID: 30282829 Free PMC article.
-
TLR1/2 Agonist Enhances Reversal of HIV-1 Latency and Promotes NK Cell-Induced Suppression of HIV-1-Infected Autologous CD4+ T Cells.J Virol. 2021 Aug 10;95(17):e0081621. doi: 10.1128/JVI.00816-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34133900 Free PMC article.
-
Targeting Cellular and Tissue HIV Reservoirs With Toll-Like Receptor Agonists.Front Immunol. 2019 Oct 15;10:2450. doi: 10.3389/fimmu.2019.02450. eCollection 2019. Front Immunol. 2019. PMID: 31681325 Free PMC article. Review.
-
Efficient Non-Epigenetic Activation of HIV Latency through the T-Cell Receptor Signalosome.Viruses. 2020 Aug 8;12(8):868. doi: 10.3390/v12080868. Viruses. 2020. PMID: 32784426 Free PMC article. Review.
Cited by
-
Knowledge From London and Berlin: Finding Threads to a Functional HIV Cure.Front Immunol. 2021 May 27;12:688747. doi: 10.3389/fimmu.2021.688747. eCollection 2021. Front Immunol. 2021. PMID: 34122453 Free PMC article. Review.
-
Modeling HIV-1 Latency in Primary T Cells Using a Replication-Competent Virus.AIDS Res Hum Retroviruses. 2016 Feb;32(2):187-93. doi: 10.1089/aid.2015.0106. Epub 2015 Jul 14. AIDS Res Hum Retroviruses. 2016. PMID: 26171776 Free PMC article.
-
A New Quinoline BRD4 Inhibitor Targets a Distinct Latent HIV-1 Reservoir for Reactivation from Other "Shock" Drugs.J Virol. 2018 Apr 27;92(10):e02056-17. doi: 10.1128/JVI.02056-17. Print 2018 May 15. J Virol. 2018. PMID: 29343578 Free PMC article.
-
Toll-like receptor 3 activation selectively reverses HIV latency in microglial cells.Retrovirology. 2017 Feb 6;14(1):9. doi: 10.1186/s12977-017-0335-8. Retrovirology. 2017. PMID: 28166799 Free PMC article.
-
Variants in the non-coding region of the TLR2 gene associated with infectious subphenotypes in pediatric sickle cell anemia.Immunogenetics. 2018 Jan;70(1):37-51. doi: 10.1007/s00251-017-1013-7. Epub 2017 Jun 30. Immunogenetics. 2018. PMID: 28667380
References
-
- Brenchley JM, Hill BJ, Ambrozak DR, Price DA, Guenaga FJ, Casazza JP, Kuruppu J, Yazdani J, Migueles SA, Connors M. et al.T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol. 2004;78:1160–1168. doi: 10.1128/JVI.78.3.1160-1168.2004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

