Measles virus C protein impairs production of defective copyback double-stranded viral RNA and activation of protein kinase R
- PMID: 24155404
- PMCID: PMC3911759
- DOI: 10.1128/JVI.02572-13
Measles virus C protein impairs production of defective copyback double-stranded viral RNA and activation of protein kinase R
Abstract
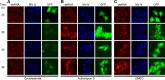
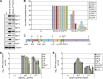
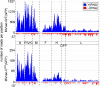
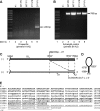
Measles virus (MV) lacking expression of C protein (C(KO)) is a potent activator of the double-stranded RNA (dsRNA)-dependent protein kinase (PKR), whereas the isogenic parental virus expressing C protein is not. Here, we demonstrate that significant amounts of dsRNA accumulate during C(KO) mutant infection but not following parental virus infection. dsRNA accumulated during late stages of infection and localized with virus replication sites containing N and P proteins. PKR autophosphorylation and stress granule formation correlated with the timing of dsRNA appearance. Phospho-PKR localized to dsRNA-containing structures as revealed by immunofluorescence. Production of dsRNA was sensitive to cycloheximide but resistant to actinomycin D, suggesting that dsRNA is a viral product. Quantitative PCR (qPCR) analyses revealed reduced viral RNA synthesis and a steepened transcription gradient in C(KO) virus-infected cells compared to those in parental virus-infected cells. The observed alterations were further reflected in lower viral protein expression levels and reduced C(KO) virus infectious yield. RNA deep sequencing confirmed the viral RNA expression profile differences seen by qPCR between C(KO) mutant and parental viruses. After one subsequent passage of the C(KO) virus, defective interfering RNA (DI-RNA) with a duplex structure was obtained that was not seen with the parental virus. We conclude that in the absence of C protein, the amount of PKR activator RNA, including DI-RNA, is increased, thereby triggering innate immune responses leading to impaired MV growth.
Figures
Similar articles
-
Measles Virus Defective Interfering RNAs Are Generated Frequently and Early in the Absence of C Protein and Can Be Destabilized by Adenosine Deaminase Acting on RNA-1-Like Hypermutations.J Virol. 2015 Aug;89(15):7735-47. doi: 10.1128/JVI.01017-15. Epub 2015 May 13. J Virol. 2015. PMID: 25972541 Free PMC article.
-
Opposing Roles of Double-Stranded RNA Effector Pathways and Viral Defense Proteins Revealed with CRISPR-Cas9 Knockout Cell Lines and Vaccinia Virus Mutants.J Virol. 2016 Aug 12;90(17):7864-79. doi: 10.1128/JVI.00869-16. Print 2016 Sep 1. J Virol. 2016. PMID: 27334583 Free PMC article.
-
The Leader Protein of Theiler's Virus Prevents the Activation of PKR.J Virol. 2019 Sep 12;93(19):e01010-19. doi: 10.1128/JVI.01010-19. Print 2019 Oct 1. J Virol. 2019. PMID: 31292248 Free PMC article.
-
Protein kinase R and the inflammasome.J Interferon Cytokine Res. 2014 Jun;34(6):447-54. doi: 10.1089/jir.2014.0008. J Interferon Cytokine Res. 2014. PMID: 24905201 Review.
-
Viral proteins targeting host protein kinase R to evade an innate immune response: a mini review.Biotechnol Genet Eng Rev. 2018 Apr;34(1):33-59. doi: 10.1080/02648725.2018.1467151. Epub 2018 May 2. Biotechnol Genet Eng Rev. 2018. PMID: 29716441 Review.
Cited by
-
Double-Stranded RNA Is Detected by Immunofluorescence Analysis in RNA and DNA Virus Infections, Including Those by Negative-Stranded RNA Viruses.J Virol. 2015 Sep;89(18):9383-92. doi: 10.1128/JVI.01299-15. Epub 2015 Jul 1. J Virol. 2015. PMID: 26136565 Free PMC article.
-
Dance with the Devil: Stress Granules and Signaling in Antiviral Responses.Viruses. 2020 Sep 4;12(9):984. doi: 10.3390/v12090984. Viruses. 2020. PMID: 32899736 Free PMC article. Review.
-
ADAR2 Is Involved in Self and Nonself Recognition of Borna Disease Virus Genomic RNA in the Nucleus.J Virol. 2020 Feb 28;94(6):e01513-19. doi: 10.1128/JVI.01513-19. Print 2020 Feb 28. J Virol. 2020. PMID: 31852792 Free PMC article.
-
Measles Virus-Induced Host Immunity and Mechanisms of Viral Evasion.Viruses. 2022 Nov 26;14(12):2641. doi: 10.3390/v14122641. Viruses. 2022. PMID: 36560645 Free PMC article. Review.
-
Upon Infection, Cellular WD Repeat-Containing Protein 5 (WDR5) Localizes to Cytoplasmic Inclusion Bodies and Enhances Measles Virus Replication.J Virol. 2018 Feb 12;92(5):e01726-17. doi: 10.1128/JVI.01726-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29237839 Free PMC article.
References
-
- Griffin DE. 2007. Measles virus, p 1551–1585 In Knipe DM, Howley PM. (ed), Fields virology, 5 ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA
-
- Cattaneo R, McChesney M. 2008. Measles virus, p 285–291 In Mahy BWJ, van Regenmortel MHV. (ed), Encyclopedia of virology, 3rd ed. Academic Press, Oxford, United Kingdom
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

