Epithelial-mesenchymal plasticity in carcinoma metastasis
- PMID: 24142872
- PMCID: PMC3814640
- DOI: 10.1101/gad.225334.113
Epithelial-mesenchymal plasticity in carcinoma metastasis
Abstract
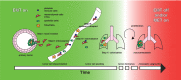
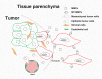
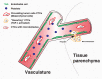
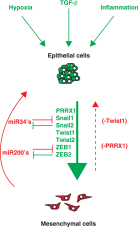
Tumor metastasis is a multistep process by which tumor cells disseminate from their primary site and form secondary tumors at a distant site. Metastasis occurs through a series of steps: local invasion, intravasation, transport, extravasation, and colonization. A developmental program termed epithelial-mesenchymal transition (EMT) has been shown to play a critical role in promoting metastasis in epithelium-derived carcinoma. Recent experimental and clinical studies have improved our knowledge of this dynamic program and implicated EMT and its reverse program, mesenchymal-epithelial transition (MET), in the metastatic process. Here, we review the functional requirement of EMT and/or MET during the individual steps of tumor metastasis and discuss the potential of targeting this program when treating metastatic diseases.
Keywords: carcinoma metastasis; epithelial–mesenchymal transition (EMT); extravasation; intravasation; invasion; mesenchymal–epithelial transition (MET); tumor dormancy.
Figures
Similar articles
-
The EMT universe: space between cancer cell dissemination and metastasis initiation.Crit Rev Oncog. 2014;19(5):349-61. doi: 10.1615/critrevoncog.2014011802. Crit Rev Oncog. 2014. PMID: 25404150 Review.
-
Epithelial-mesenchymal transition in colorectal cancer metastasis: A system review.Pathol Res Pract. 2015 Aug;211(8):557-69. doi: 10.1016/j.prp.2015.05.010. Epub 2015 May 28. Pathol Res Pract. 2015. PMID: 26092594 Review.
-
Epigenetic Regulation of Epithelial to Mesenchymal Transition in the Cancer Metastatic Cascade: Implications for Cancer Therapy.Front Oncol. 2021 Apr 29;11:657546. doi: 10.3389/fonc.2021.657546. eCollection 2021. Front Oncol. 2021. PMID: 33996581 Free PMC article. Review.
-
Epithelial Mesenchymal Transition in Tumor Metastasis.Annu Rev Pathol. 2018 Jan 24;13:395-412. doi: 10.1146/annurev-pathol-020117-043854. Annu Rev Pathol. 2018. PMID: 29414248 Review.
-
The lncRNA H19 mediates breast cancer cell plasticity during EMT and MET plasticity by differentially sponging miR-200b/c and let-7b.Sci Signal. 2017 Jun 13;10(483):eaak9557. doi: 10.1126/scisignal.aak9557. Sci Signal. 2017. PMID: 28611183
Cited by
-
PAD4 and Its Inhibitors in Cancer Progression and Prognosis.Pharmaceutics. 2022 Nov 8;14(11):2414. doi: 10.3390/pharmaceutics14112414. Pharmaceutics. 2022. PMID: 36365233 Free PMC article. Review.
-
Insulin-like growth factor binding protein 5 (IGFBP5) functions as a tumor suppressor in human melanoma cells.Oncotarget. 2015 Aug 21;6(24):20636-49. doi: 10.18632/oncotarget.4114. Oncotarget. 2015. PMID: 26010068 Free PMC article.
-
STYK1 promotes epithelial-mesenchymal transition and tumor metastasis in human hepatocellular carcinoma through MEK/ERK and PI3K/AKT signaling.Sci Rep. 2016 Sep 15;6:33205. doi: 10.1038/srep33205. Sci Rep. 2016. PMID: 27628214 Free PMC article.
-
FOXC2 regulates the G2/M transition of stem cell-rich breast cancer cells and sensitizes them to PLK1 inhibition.Sci Rep. 2016 Apr 11;6:23070. doi: 10.1038/srep23070. Sci Rep. 2016. PMID: 27064522 Free PMC article.
-
Modulating barriers of tumor microenvironment through nanocarrier systems for improved cancer immunotherapy: a review of current status and future perspective.Drug Deliv. 2020 Dec;27(1):1248-1262. doi: 10.1080/10717544.2020.1809559. Drug Deliv. 2020. PMID: 32865029 Free PMC article. Review.
References
-
- Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S, et al. 2008. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell 14: 79–89 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
