Glutaminase regulation in cancer cells: a druggable chain of events
- PMID: 24140288
- PMCID: PMC3989473
- DOI: 10.1016/j.drudis.2013.10.008
Glutaminase regulation in cancer cells: a druggable chain of events
Abstract
Metabolism is the process by which cells convert relatively simple extracellular nutrients into energy and building blocks necessary for their growth and survival. In cancer cells, metabolism is dramatically altered compared with normal cells. These alterations are known as the Warburg effect. One consequence of these changes is cellular addiction to glutamine. Because of this, in recent years the enzyme glutaminase has become a key target for small molecule therapeutic intervention. Like many oncotargets, however, glutaminase has a number of upstream partners that might offer additional druggable targets. This review summarizes the work from the current decade surrounding glutaminase and its regulation, and suggests strategies for therapeutic intervention in relevant cases.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures
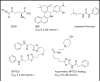

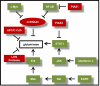
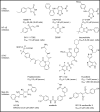
Similar articles
-
Therapeutic strategies impacting cancer cell glutamine metabolism.Future Med Chem. 2013 Sep;5(14):1685-700. doi: 10.4155/fmc.13.130. Future Med Chem. 2013. PMID: 24047273 Free PMC article. Review.
-
Therapeutic targeting of glutaminolysis as an essential strategy to combat cancer.Semin Cell Dev Biol. 2020 Feb;98:34-43. doi: 10.1016/j.semcdb.2019.05.012. Epub 2019 May 22. Semin Cell Dev Biol. 2020. PMID: 31100352 Review.
-
Recent Development of Small Molecule Glutaminase Inhibitors.Curr Top Med Chem. 2018;18(6):432-443. doi: 10.2174/1568026618666180525100830. Curr Top Med Chem. 2018. PMID: 29793408 Review.
-
Glutaminases regulate glutathione and oxidative stress in cancer.Arch Toxicol. 2020 Aug;94(8):2603-2623. doi: 10.1007/s00204-020-02838-8. Epub 2020 Jul 18. Arch Toxicol. 2020. PMID: 32681190 Review.
-
Targeting GLS1 to cancer therapy through glutamine metabolism.Clin Transl Oncol. 2021 Nov;23(11):2253-2268. doi: 10.1007/s12094-021-02645-2. Epub 2021 May 23. Clin Transl Oncol. 2021. PMID: 34023970 Review.
Cited by
-
Glutaminase Inhibition on NSCLC Depends on Extracellular Alanine Exploitation.Cells. 2020 Jul 23;9(8):1766. doi: 10.3390/cells9081766. Cells. 2020. PMID: 32718002 Free PMC article.
-
Discovery and Optimization of Ergosterol Peroxide Derivatives as Novel Glutaminase 1 Inhibitors for the Treatment of Triple-Negative Breast Cancer.Molecules. 2024 Sep 14;29(18):4375. doi: 10.3390/molecules29184375. Molecules. 2024. PMID: 39339370 Free PMC article.
-
New Avenues of Heme Synthesis Regulation.Int J Mol Sci. 2022 Jul 5;23(13):7467. doi: 10.3390/ijms23137467. Int J Mol Sci. 2022. PMID: 35806474 Free PMC article. Review.
-
Targeting glutaminase-mediated glutamine dependence in papillary thyroid cancer.J Mol Med (Berl). 2018 Aug;96(8):777-790. doi: 10.1007/s00109-018-1659-0. Epub 2018 Jun 25. J Mol Med (Berl). 2018. PMID: 29942976
-
Targeting Glutamine Induces Apoptosis: A Cancer Therapy Approach.Int J Mol Sci. 2015 Sep 22;16(9):22830-55. doi: 10.3390/ijms160922830. Int J Mol Sci. 2015. PMID: 26402672 Free PMC article. Review.
References
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
-
- Szeliga M, Obara-Michlewska M. Glutamine in neoplastic cells: focus on the expression and roles of glutaminases. Neurochem. Int. 2009;55:71–75. - PubMed
-
- Aledo JC, et al. Identification of two human glutaminase loci and tissue-specific expression of the two related genes. Mamm. Genome. 2000;11:1107–1110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

