CDIP1-BAP31 complex transduces apoptotic signals from endoplasmic reticulum to mitochondria under endoplasmic reticulum stress
- PMID: 24139803
- PMCID: PMC3833439
- DOI: 10.1016/j.celrep.2013.09.020
CDIP1-BAP31 complex transduces apoptotic signals from endoplasmic reticulum to mitochondria under endoplasmic reticulum stress
Abstract
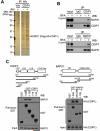
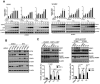
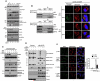
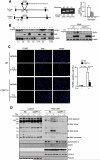
Resolved endoplasmic reticulum (ER) stress response is essential for intracellular homeostatic balance, but unsettled ER stress can lead to apoptosis. Here, we show that a proapoptotic p53 target, CDIP1, acts as a key signal transducer of ER-stress-mediated apoptosis. We identify B-cell-receptor-associated protein 31 (BAP31) as an interacting partner of CDIP1. Upon ER stress, CDIP1 is induced and enhances an association with BAP31 at the ER membrane. We also show that CDIP1 binding to BAP31 is required for BAP31 cleavage upon ER stress and for BAP31-Bcl-2 association. The recruitment of Bcl-2 to the BAP31-CDIP1 complex, as well as CDIP1-dependent truncated Bid (tBid) and caspase-8 activation, contributes to BAX oligomerization. Genetic knockout of CDIP1 in mice leads to impaired response to ER-stress-mediated apoptosis. Altogether, our data demonstrate that the CDIP1/BAP31-mediated regulation of mitochondrial apoptosis pathway represents a mechanism for establishing an ER-mitochondrial crosstalk for ER-stress-mediated apoptosis signaling.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol.J Cell Biol. 2003 Mar 31;160(7):1115-27. doi: 10.1083/jcb.200212059. J Cell Biol. 2003. PMID: 12668660 Free PMC article.
-
Fis1 and Bap31 bridge the mitochondria-ER interface to establish a platform for apoptosis induction.EMBO J. 2011 Feb 2;30(3):556-68. doi: 10.1038/emboj.2010.346. Epub 2010 Dec 24. EMBO J. 2011. PMID: 21183955 Free PMC article.
-
Uncleaved BAP31 in association with A4 protein at the endoplasmic reticulum is an inhibitor of Fas-initiated release of cytochrome c from mitochondria.J Biol Chem. 2003 Apr 18;278(16):14461-8. doi: 10.1074/jbc.M209684200. Epub 2003 Jan 15. J Biol Chem. 2003. PMID: 12529377
-
Regulation of endoplasmic reticulum Ca2+ dynamics by proapoptotic BCL-2 family members.Biochem Pharmacol. 2003 Oct 15;66(8):1335-40. doi: 10.1016/s0006-2952(03)00482-9. Biochem Pharmacol. 2003. PMID: 14555206 Review.
-
New insights in the role of Bcl-2 Bcl-2 and the endoplasmic reticulum.Apoptosis. 2002 Oct;7(5):441-7. doi: 10.1023/a:1020087108926. Apoptosis. 2002. PMID: 12207177 Review.
Cited by
-
Endoplasmic Reticulum-Mitochondria Contact Sites and Neurodegeneration.Front Cell Dev Biol. 2020 Jun 18;8:428. doi: 10.3389/fcell.2020.00428. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32626703 Free PMC article. Review.
-
Mitochondria-Associated Endoplasmic Reticulum Membranes: Inextricably Linked with Autophagy Process.Oxid Med Cell Longev. 2022 Aug 23;2022:7086807. doi: 10.1155/2022/7086807. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36052160 Free PMC article. Review.
-
Induction of Liver Steatosis in BAP31-Deficient Mice Burdened with Tunicamycin-Induced Endoplasmic Reticulum Stress.Int J Mol Sci. 2018 Aug 4;19(8):2291. doi: 10.3390/ijms19082291. Int J Mol Sci. 2018. PMID: 30081561 Free PMC article.
-
Mitochondria-Associated Endoplasmic Reticulum Membranes (MAMs) and Their Prospective Roles in Kidney Disease.Oxid Med Cell Longev. 2020 Sep 3;2020:3120539. doi: 10.1155/2020/3120539. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32952849 Free PMC article. Review.
-
Metabolic implications of organelle-mitochondria communication.EMBO Rep. 2019 Sep;20(9):e47928. doi: 10.15252/embr.201947928. Epub 2019 Aug 14. EMBO Rep. 2019. PMID: 31418169 Free PMC article. Review.
References
-
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

