Physiological roles of aquaporin-4 in brain
- PMID: 24137016
- PMCID: PMC3858210
- DOI: 10.1152/physrev.00011.2013
Physiological roles of aquaporin-4 in brain
Abstract
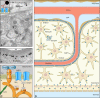
Aquaporin-4 (AQP4) is one of the most abundant molecules in the brain and is particularly prevalent in astrocytic membranes at the blood-brain and brain-liquor interfaces. While AQP4 has been implicated in a number of pathophysiological processes, its role in brain physiology has remained elusive. Only recently has evidence accumulated to suggest that AQP4 is involved in such diverse functions as regulation of extracellular space volume, potassium buffering, cerebrospinal fluid circulation, interstitial fluid resorption, waste clearance, neuroinflammation, osmosensation, cell migration, and Ca(2+) signaling. AQP4 is also required for normal function of the retina, inner ear, and olfactory system. A review will be provided of the physiological roles of AQP4 in brain and of the growing list of data that emphasize the polarized nature of astrocytes.
Figures



Similar articles
-
Aquaporin 4 in Astrocytes is a Target for Therapy in Alzheimer's Disease.Curr Pharm Des. 2017;23(33):4948-4957. doi: 10.2174/1381612823666170714144844. Curr Pharm Des. 2017. PMID: 28714415 Review.
-
Insights into Cell Surface Expression, Supramolecular Organization, and Functions of Aquaporin 4 Isoforms in Astrocytes.Cells. 2020 Dec 7;9(12):2622. doi: 10.3390/cells9122622. Cells. 2020. PMID: 33297299 Free PMC article. Review.
-
Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport.Neuroscience. 2004;129(4):999-1010. doi: 10.1016/j.neuroscience.2004.08.049. Neuroscience. 2004. PMID: 15561415 Review.
-
Aquaporins in the brain: from aqueduct to "multi-duct".Metab Brain Dis. 2007 Dec;22(3-4):251-63. doi: 10.1007/s11011-007-9057-2. Metab Brain Dis. 2007. PMID: 17701333 Review.
-
Three distinct roles of aquaporin-4 in brain function revealed by knockout mice.Biochim Biophys Acta. 2006 Aug;1758(8):1085-93. doi: 10.1016/j.bbamem.2006.02.018. Epub 2006 Mar 10. Biochim Biophys Acta. 2006. PMID: 16564496 Review.
Cited by
-
The pathogenesis of idiopathic normal pressure hydrocephalus based on the understanding of AQP1 and AQP4.Front Mol Neurosci. 2022 Sep 20;15:952036. doi: 10.3389/fnmol.2022.952036. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36204139 Free PMC article. Review.
-
Factors determining the density of AQP4 water channel molecules at the brain-blood interface.Brain Struct Funct. 2017 May;222(4):1753-1766. doi: 10.1007/s00429-016-1305-y. Epub 2016 Sep 15. Brain Struct Funct. 2017. PMID: 27629271 Free PMC article.
-
Emerging Role of Neuron-Glia in Neurological Disorders: At a Glance.Oxid Med Cell Longev. 2022 Aug 22;2022:3201644. doi: 10.1155/2022/3201644. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36046684 Free PMC article. Review.
-
Astrocyte Activation in Neurovascular Damage and Repair Following Ischaemic Stroke.Int J Mol Sci. 2021 Apr 20;22(8):4280. doi: 10.3390/ijms22084280. Int J Mol Sci. 2021. PMID: 33924191 Free PMC article. Review.
-
Revisiting Astrocytic Roles in Methylmercury Intoxication.Mol Neurobiol. 2021 Sep;58(9):4293-4308. doi: 10.1007/s12035-021-02420-y. Epub 2021 May 14. Mol Neurobiol. 2021. PMID: 33990914 Review.
References
-
- Alvestad S, Hammer J, Hoddevik EH, Skare O, Sonnewald U, Amiry-Moghaddam M, Ottersen OP. Mislocalization of AQP4 precedes chronic seizures in the kainate model of temporal lobe epilepsy. Epilepsy Res 105: 30–41, 2013 - PubMed
-
- Amiry-Moghaddam M, Otsuka T, Hurn PD, Traystman RJ, Haug FM, Froehner SC, Adams ME, Neely JD, Agre P, Ottersen OP, Bhardwaj A. An alpha-syntrophin-dependent pool of AQP4 in astroglial end-feet confers bidirectional water flow between blood and brain. Proc Natl Acad Sci USA 100: 2106–2111, 2003 - PMC - PubMed
-
- Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci 4: 991–1001, 2003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

