WT1 maintains adrenal-gonadal primordium identity and marks a population of AGP-like progenitors within the adrenal gland
- PMID: 24135228
- PMCID: PMC4032791
- DOI: 10.1016/j.devcel.2013.09.003
WT1 maintains adrenal-gonadal primordium identity and marks a population of AGP-like progenitors within the adrenal gland
Abstract
Adrenal glands and gonads share a common primordium (AGP), but the molecular events driving differentiation are poorly understood. Here we demonstrate that the Wilms tumor suppressor WT1 is a key factor defining AGP identity by inhibiting the steroidogenic differentiation process. Indeed, ectopic expression of WT1 precludes differentiation into adrenocortical steroidogenic cells by locking them into a progenitor state. Chromatin immunoprecipitation experiments identify Tcf21 and Gli1 as direct targets of WT1. Moreover, cell lineage tracing analyses identify a long-living progenitor population within the adrenal gland, characterized by the expression of WT1, GATA4, GLI1, and TCF21, that can generate steroidogenic cells in vivo. Strikingly, gonadectomy dramatically activates these WT1(+) cells and leads to their differentiation into gonadal steroidogenic tissue. Thus, our data describe a mechanism of response to organ loss by recreating hormone-producing cells at a heterotopic site.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

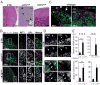
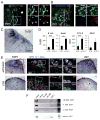
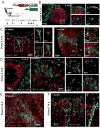
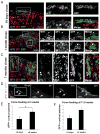

Comment in
-
Never underestimate the complexity of remodeling.Endocrinology. 2013 Dec;154(12):4446-9. doi: 10.1210/en.2013-1982. Endocrinology. 2013. PMID: 24273232 No abstract available.
Similar articles
-
GLI1+ progenitor cells in the adrenal capsule of the adult mouse give rise to heterotopic gonadal-like tissue.Mol Cell Endocrinol. 2017 Feb 5;441:164-175. doi: 10.1016/j.mce.2016.08.043. Epub 2016 Aug 29. Mol Cell Endocrinol. 2017. PMID: 27585489 Free PMC article.
-
Never underestimate the complexity of remodeling.Endocrinology. 2013 Dec;154(12):4446-9. doi: 10.1210/en.2013-1982. Endocrinology. 2013. PMID: 24273232 No abstract available.
-
Steroidogenic organ development and homeostasis: A WT1-centric view.Mol Cell Endocrinol. 2015 Jun 15;408:145-55. doi: 10.1016/j.mce.2015.01.009. Epub 2015 Jan 14. Mol Cell Endocrinol. 2015. PMID: 25596547 Review.
-
Adrenal development is initiated by Cited2 and Wt1 through modulation of Sf-1 dosage.Development. 2007 Jun;134(12):2349-58. doi: 10.1242/dev.004390. Development. 2007. PMID: 17537799
-
Toying with fate: Redirecting the differentiation of adrenocortical progenitor cells into gonadal-like tissue.Mol Cell Endocrinol. 2015 Jun 15;408:165-77. doi: 10.1016/j.mce.2014.12.003. Epub 2014 Dec 8. Mol Cell Endocrinol. 2015. PMID: 25498963 Free PMC article. Review.
Cited by
-
Novel markers of gonadectomy-induced adrenocortical neoplasia in the mouse and ferret.Mol Cell Endocrinol. 2015 Jan 5;399:122-30. doi: 10.1016/j.mce.2014.09.029. Epub 2014 Oct 5. Mol Cell Endocrinol. 2015. PMID: 25289806 Free PMC article.
-
Stem cell function and plasticity in the normal physiology of the adrenal cortex.Mol Cell Endocrinol. 2021 Jan 1;519:111043. doi: 10.1016/j.mce.2020.111043. Epub 2020 Oct 12. Mol Cell Endocrinol. 2021. PMID: 33058950 Free PMC article. Review.
-
The adrenal capsule is a signaling center controlling cell renewal and zonation through Rspo3.Genes Dev. 2016 Jun 15;30(12):1389-94. doi: 10.1101/gad.277756.116. Epub 2016 Jun 16. Genes Dev. 2016. PMID: 27313319 Free PMC article.
-
Wilms' tumor 1 drives fibroproliferation and myofibroblast transformation in severe fibrotic lung disease.JCI Insight. 2018 Aug 23;3(16):e121252. doi: 10.1172/jci.insight.121252. eCollection 2018 Aug 23. JCI Insight. 2018. PMID: 30135315 Free PMC article.
-
Evaluating the role of aldosterone synthesis on adrenal cell fate.Front Endocrinol (Lausanne). 2024 Aug 7;15:1423027. doi: 10.3389/fendo.2024.1423027. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39170743 Free PMC article. Review.
References
-
- Anderson DJ, Axel R. A bipotential neuroendocrine precursor whose choice of cell fate is determined by NGF and glucocorticoids. Cell. 1986;47:1079–1090. - PubMed
-
- Bickmore WA, Oghene K, Little MH, Seawright A, van Heyningen V, Hastie ND. Modulation of DNA binding specificity by alternative splicing of the Wilms tumor wt1 gene transcript. Science. 1992;257:235–237. - PubMed
-
- Bielinska M, Genova E, Boime I, Parviainen H, Kiiveri S, Leppaluoto J, Rahman N, Heikinheimo M, Wilson DB. Gonadotropin-induced adrenocortical neoplasia in NU/J nude mice. Endocrinology. 2005;146:3975–3984. - PubMed
-
- Bielinska M, Parviainen H, Porter-Tinge SB, Kiiveri S, Genova E, Rahman N, Huhtaniemi IT, Muglia LJ, Heikinheimo M, Wilson DB. Mouse strain susceptibility to gonadectomy-induced adrenocortical tumor formation correlates with the expression of GATA-4 and luteinizing hormone receptor. Endocrinology. 2003;144:4123–4133. - PubMed
-
- Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44:419–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

