Human cytomegalovirus latency-associated proteins elicit immune-suppressive IL-10 producing CD4⁺ T cells
- PMID: 24130479
- PMCID: PMC3795018
- DOI: 10.1371/journal.ppat.1003635
Human cytomegalovirus latency-associated proteins elicit immune-suppressive IL-10 producing CD4⁺ T cells
Abstract
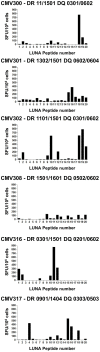
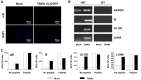
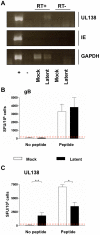
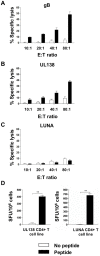
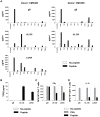
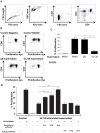
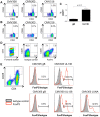
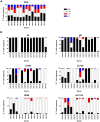
Human cytomegalovirus (HCMV) is a widely prevalent human herpesvirus, which, after primary infection, persists in the host for life. In healthy individuals, the virus is well controlled by the HCMV-specific T cell response. A key feature of this persistence, in the face of a normally robust host immune response, is the establishment of viral latency. In contrast to lytic infection, which is characterised by extensive viral gene expression and virus production, long-term latency in cells of the myeloid lineage is characterised by highly restricted expression of viral genes, including UL138 and LUNA. Here we report that both UL138 and LUNA-specific T cells were detectable directly ex vivo in healthy HCMV seropositive subjects and that this response is principally CD4⁺ T cell mediated. These UL138-specific CD4⁺ T cells are able to mediate MHC class II restricted cytotoxicity and, importantly, show IFNγ effector function in the context of both lytic and latent infection. Furthermore, in contrast to CDCD4⁺ T cells specific to antigens expressed solely during lytic infection, both the UL138 and LUNA-specific CD4⁺ T cell responses included CD4⁺ T cells that secreted the immunosuppressive cytokine cIL-10. We also show that cIL-10 expressing CD4⁺ T-cells are directed against latently expressed US28 and UL111A. Taken together, our data show that latency-associated gene products of HCMV generate CD4⁺ T cell responses in vivo, which are able to elicit effector function in response to both lytic and latently infected cells. Importantly and in contrast to CD4⁺ T cell populations, which recognise antigens solely expressed during lytic infection, include a subset of cells that secrete the immunosuppressive cytokine cIL-10. This suggests that HCMV skews the T cell responses to latency-associated antigens to one that is overall suppressive in order to sustain latent carriage in vivo.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Latency-Associated Expression of Human Cytomegalovirus US28 Attenuates Cell Signaling Pathways To Maintain Latent Infection.mBio. 2017 Dec 5;8(6):e01754-17. doi: 10.1128/mBio.01754-17. mBio. 2017. PMID: 29208743 Free PMC article.
-
The role of the human cytomegalovirus UL111A gene in down-regulating CD4+ T-cell recognition of latently infected cells: implications for virus elimination during latency.Blood. 2009 Nov 5;114(19):4128-37. doi: 10.1182/blood-2008-12-197111. Epub 2009 Aug 25. Blood. 2009. PMID: 19706889
-
Human Cytomegalovirus US28 Ligand Binding Activity Is Required for Latency in CD34+ Hematopoietic Progenitor Cells and Humanized NSG Mice.mBio. 2019 Aug 20;10(4):e01889-19. doi: 10.1128/mBio.01889-19. mBio. 2019. PMID: 31431555 Free PMC article.
-
Sleepless latency of human cytomegalovirus.Med Microbiol Immunol. 2015 Jun;204(3):421-9. doi: 10.1007/s00430-015-0401-6. Epub 2015 Mar 14. Med Microbiol Immunol. 2015. PMID: 25772624 Free PMC article. Review.
-
Human Cytomegalovirus Interleukin 10 Homologs: Facing the Immune System.Front Cell Infect Microbiol. 2020 Jun 9;10:245. doi: 10.3389/fcimb.2020.00245. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32582563 Free PMC article. Review.
Cited by
-
Current approaches used in treating COVID-19 from a molecular mechanisms and immune response perspective.Saudi Pharm J. 2020 Nov;28(11):1333-1352. doi: 10.1016/j.jsps.2020.08.024. Epub 2020 Sep 1. Saudi Pharm J. 2020. PMID: 32905015 Free PMC article. Review.
-
Exploiting viral natural history for vaccine development.Med Microbiol Immunol. 2015 Jun;204(3):255-62. doi: 10.1007/s00430-015-0406-1. Epub 2015 Mar 21. Med Microbiol Immunol. 2015. PMID: 25794555 Free PMC article. Review.
-
IL-10-Secreting CD8+ T Cells Specific for Human Cytomegalovirus (HCMV): Generation, Maintenance and Phenotype.Pathogens. 2022 Dec 13;11(12):1530. doi: 10.3390/pathogens11121530. Pathogens. 2022. PMID: 36558866 Free PMC article.
-
Impact of Cytomegalovirus Disease on New-Onset Type 2 Diabetes Mellitus: Population-Based Matched Case-Control Cohort Study.Diabetes Metab J. 2019 Dec;43(6):815-829. doi: 10.4093/dmj.2018.0167. Epub 2019 Jan 21. Diabetes Metab J. 2019. PMID: 30688050 Free PMC article.
-
Cytomegalovirus Latency and Reactivation: An Intricate Interplay With the Host Immune Response.Front Cell Infect Microbiol. 2020 Mar 31;10:130. doi: 10.3389/fcimb.2020.00130. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32296651 Free PMC article. Review.
References
-
- Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, et al. (2006) Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis 43: 1143–1151. - PubMed
-
- Jackson SE, Mason GM, Wills MR (2011) Human cytomegalovirus immunity and immune evasion. Virus Research 157: 151–160. - PubMed
-
- Wills MR, Carmichael AJ, Sissons JG, editors(2006) Adaptive cellular immunity to human cytomegalovirus. 1st ed. Wymondham: Caister Academic press. 25 p.
-
- Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, et al. (2002) Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 99: 3916–3922. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

