MAS promoter regulation: a role for Sry and tyrosine nitration of the KRAB domain of ZNF274 as a feedback mechanism
- PMID: 24128372
- PMCID: PMC4255987
- DOI: 10.1042/CS20130385
MAS promoter regulation: a role for Sry and tyrosine nitration of the KRAB domain of ZNF274 as a feedback mechanism
Abstract
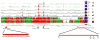
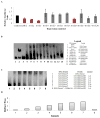
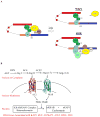
The ACE2 (angiotensin-converting enzyme 2)/Ang-(1-7) [angiotensin-(1-7)]/MAS axis of the RAS (renin-angiotensin system) has emerged as a pathway of interest in treating both cardiovascular disorders and cancer. The MAS protein is known to bind to and be activated by Ang-(1-7); however, the mechanisms of this activation are just starting to be understood. Although there are strong biochemical data regarding the regulation and activation of the AT1R (angiotensin II type 1 receptor) and the AT2R (angiotensin II type 2 receptor), with models of how AngII (angiotensin II) binds each receptor, fewer studies have characterized MAS. In the present study, we characterize the MAS promoter and provide a potential feedback mechanism that could compensate for MAS degradation following activation by Ang-(1-7). Analysis of ENCODE data for the MAS promoter revealed potential epigenetic control by KRAB (Krüppel-associated box)/KAP-1 (KRAB-associated protein-1). A proximal promoter construct for the MAS gene was repressed by the SOX [SRY (sex-determining region on the Y chromosome) box] proteins SRY, SOX2, SOX3 and SOX14, of which SRY is known to interact with the KRAB domain. The KRAB-KAP-1 complex can be tyrosine-nitrated, causing the dissociation of the KAP-1 protein and thus a potential loss of epigenetic control. Activation of MAS can lead to an increase in nitric oxide, suggesting a feedback mechanism for MAS on its own promoter. The results of the present study provide a more complete view of MAS regulation and, for the first time, suggest biochemical outcomes for nitration of the KRAB domain.
Figures
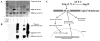

Similar articles
-
Similarities and differences of X and Y chromosome homologous genes, SRY and SOX3, in regulating the renin-angiotensin system promoters.Physiol Genomics. 2015 May;47(5):177-86. doi: 10.1152/physiolgenomics.00138.2014. Epub 2015 Mar 10. Physiol Genomics. 2015. PMID: 25759379 Free PMC article.
-
Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis protects against lung fibrosis by inhibiting the MAPK/NF-κB pathway.Am J Respir Cell Mol Biol. 2014 Apr;50(4):723-36. doi: 10.1165/rcmb.2012-0451OC. Am J Respir Cell Mol Biol. 2014. PMID: 24168260
-
The renin-angiotensin system and its vasoactive metabolite angiotensin-(1-7) in the mechanism of the healing of preexisting gastric ulcers. The involvement of Mas receptors, nitric oxide, prostaglandins and proinflammatory cytokines.J Physiol Pharmacol. 2016 Feb;67(1):75-91. J Physiol Pharmacol. 2016. PMID: 27010897
-
Devil and angel in the renin-angiotensin system: ACE-angiotensin II-AT1 receptor axis vs. ACE2-angiotensin-(1-7)-Mas receptor axis.Hypertens Res. 2009 Jul;32(7):533-6. doi: 10.1038/hr.2009.74. Epub 2009 May 22. Hypertens Res. 2009. PMID: 19461648 Free PMC article. Review.
-
Physical Exercise and ACE2-Angiotensin-(1-7)-Mas Receptor Axis of the Renin Angiotensin System.Protein Pept Lett. 2017 Nov 17;24(9):809-816. doi: 10.2174/0929866524666170728151401. Protein Pept Lett. 2017. PMID: 28758593 Review.
Cited by
-
Sex Drives Dimorphic Immune Responses to Viral Infections.J Immunol. 2017 Mar 1;198(5):1782-1790. doi: 10.4049/jimmunol.1601166. J Immunol. 2017. PMID: 28223406 Free PMC article. Review.
-
Chromosome Y genetic variants: impact in animal models and on human disease.Physiol Genomics. 2015 Nov;47(11):525-37. doi: 10.1152/physiolgenomics.00074.2015. Epub 2015 Aug 18. Physiol Genomics. 2015. PMID: 26286457 Free PMC article. Review.
-
Similarities and differences of X and Y chromosome homologous genes, SRY and SOX3, in regulating the renin-angiotensin system promoters.Physiol Genomics. 2015 May;47(5):177-86. doi: 10.1152/physiolgenomics.00138.2014. Epub 2015 Mar 10. Physiol Genomics. 2015. PMID: 25759379 Free PMC article.
-
Expression of the Mas receptor is upregulated in skeletal muscle wasting.Histochem Cell Biol. 2015 Feb;143(2):131-41. doi: 10.1007/s00418-014-1275-1. Epub 2014 Sep 11. Histochem Cell Biol. 2015. PMID: 25208653
References
-
- Young D, Waitches G, Birchmeier C, Fasano O, Wigler M. Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell. 1986;45:711–719. - PubMed
-
- Jackson TR, Blair LA, Marshall J, Goedert M, Hanley MR. The mas oncogene encodes an angiotensin receptor. Nature. 1988;335:437–440. - PubMed
-
- Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M, Mendes EP, Lemos VS, Campagnole-Santos MJ, Schultheiss HP, Speth R, Walther T. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. - PMC - PubMed
-
- Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49:185–192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

