The role of transmembrane channel-like proteins in the operation of hair cell mechanotransducer channels
- PMID: 24127526
- PMCID: PMC3813385
- DOI: 10.1085/jgp.201311068
The role of transmembrane channel-like proteins in the operation of hair cell mechanotransducer channels
Abstract
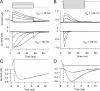
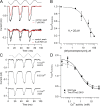
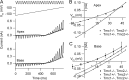
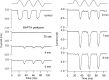
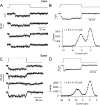
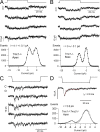
Sound stimuli elicit movement of the stereocilia that make up the hair bundle of cochlear hair cells, putting tension on the tip links connecting the stereocilia and thereby opening mechanotransducer (MT) channels. Tmc1 and Tmc2, two members of the transmembrane channel-like family, are necessary for mechanotransduction. To assess their precise role, we recorded MT currents elicited by hair bundle deflections in mice with null mutations of Tmc1, Tmc2, or both. During the first postnatal week, we observed a normal MT current in hair cells lacking Tmc1 or Tmc2; however, in the absence of both isoforms, we recorded a large MT current that was phase-shifted 180°, being evoked by displacements of the hair bundle away from its tallest edge rather than toward it as in wild-type hair cells. The anomalous MT current in hair cells lacking Tmc1 and Tmc2 was blocked by FM1-43, dihydrostreptomycin, and extracellular Ca(2+) at concentrations similar to those that blocked wild type. MT channels in the double knockouts carried Ca(2+) with a lower permeability than wild-type or single mutants. The MT current in double knockouts persisted during exposure to submicromolar Ca(2+), even though this treatment destroyed the tip links. We conclude that the Tmc isoforms do not themselves constitute the MT channel but are essential for targeting and interaction with the tip link. Changes in the MT conductance and Ca(2+) permeability observed in the absence of Tmc1 mutants may stem from loss of interaction with protein partners in the transduction complex.
Figures



Comment in
-
Who needs tip links? Backwards transduction by hair cells.J Gen Physiol. 2013 Nov;142(5):481-6. doi: 10.1085/jgp.201311111. Epub 2013 Oct 14. J Gen Physiol. 2013. PMID: 24127528 Free PMC article. No abstract available.
Similar articles
-
Conductance and block of hair-cell mechanotransducer channels in transmembrane channel-like protein mutants.J Gen Physiol. 2014 Jul;144(1):55-69. doi: 10.1085/jgp.201411173. J Gen Physiol. 2014. PMID: 24981230 Free PMC article.
-
Developmental changes in the cochlear hair cell mechanotransducer channel and their regulation by transmembrane channel-like proteins.J Gen Physiol. 2013 Jan;141(1):141-8. doi: 10.1085/jgp.201210913. J Gen Physiol. 2013. PMID: 23277480 Free PMC article.
-
The contribution of TMC1 to adaptation of mechanoelectrical transduction channels in cochlear outer hair cells.J Physiol. 2019 Dec;597(24):5949-5961. doi: 10.1113/JP278799. Epub 2019 Nov 12. J Physiol. 2019. PMID: 31633194 Free PMC article.
-
Is TMC1 the Hair Cell Mechanotransducer Channel?Biophys J. 2016 Jul 12;111(1):3-9. doi: 10.1016/j.bpj.2016.05.032. Biophys J. 2016. PMID: 27410728 Free PMC article. Review.
-
PIEZO2 as the anomalous mechanotransducer channel in auditory hair cells.J Physiol. 2017 Dec 1;595(23):7039-7048. doi: 10.1113/JP274996. Epub 2017 Oct 26. J Physiol. 2017. PMID: 28983916 Free PMC article. Review.
Cited by
-
Genetic etiology of hereditary hearing loss in the Gulf Cooperation Council countries.Hum Genet. 2022 Apr;141(3-4):595-605. doi: 10.1007/s00439-021-02323-x. Epub 2021 Aug 2. Hum Genet. 2022. PMID: 34338889 Review.
-
The speed of the hair cell mechanotransducer channel revealed by fluctuation analysis.J Gen Physiol. 2021 Oct 4;153(10):e202112959. doi: 10.1085/jgp.202112959. Epub 2021 Aug 19. J Gen Physiol. 2021. PMID: 34411238 Free PMC article.
-
Adaptation Independent Modulation of Auditory Hair Cell Mechanotransduction Channel Open Probability Implicates a Role for the Lipid Bilayer.J Neurosci. 2016 Mar 9;36(10):2945-56. doi: 10.1523/JNEUROSCI.3011-15.2016. J Neurosci. 2016. PMID: 26961949 Free PMC article.
-
Molecular Specializations Underlying Phenotypic Differences in Inner Ear Hair Cells of Zebrafish and Mice.bioRxiv [Preprint]. 2024 May 26:2024.05.24.595729. doi: 10.1101/2024.05.24.595729. bioRxiv. 2024. Update in: Front Neurol. 2024 Oct 17;15:1437558. doi: 10.3389/fneur.2024.1437558. PMID: 38826418 Free PMC article. Updated. Preprint.
-
Mechanosensory hair cells express two molecularly distinct mechanotransduction channels.Nat Neurosci. 2017 Jan;20(1):24-33. doi: 10.1038/nn.4449. Epub 2016 Nov 28. Nat Neurosci. 2017. PMID: 27893727 Free PMC article.
References
-
- Alagramam K.N., Goodyear R.J., Geng R., Furness D.N., van Aken A.F., Marcotti W., Kros C.J., Richardson G.P. 2011. Mutations in protocadherin 15 and cadherin 23 affect tip links and mechanotransduction in mammalian sensory hair cells. PLoS ONE. 6:e19183 10.1371/journal.pone.0019183 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

