PINK1 is degraded through the N-end rule pathway
- PMID: 24121706
- PMCID: PMC4028335
- DOI: 10.4161/auto.24633
PINK1 is degraded through the N-end rule pathway
Abstract
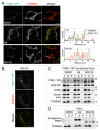
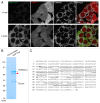
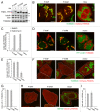
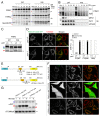
PINK1, a mitochondrial serine/threonine kinase, is the product of a gene mutated in an autosomal recessive form of Parkinson disease. PINK1 is constitutively degraded by an unknown mechanism and stabilized selectively on damaged mitochondria where it can recruit the E3 ligase PARK2/PARKIN to induce mitophagy. Here, we show that, under steady-state conditions, endogenous PINK1 is constitutively and rapidly degraded by E3 ubiquitin ligases UBR1, UBR2 and UBR4 through the N-end rule pathway. Following precursor import into mitochondria, PINK1 is cleaved in the transmembrane segment by a mitochondrial intramembrane protease PARL generating an N-terminal destabilizing amino acid and then retrotranslocates from mitochondria to the cytosol for N-end recognition and proteasomal degradation. Thus, sequential actions of mitochondrial import, PARL-processing, retrotranslocation and recognition by N-end rule E3 enzymes for the ubiquitin proteosomal degradation defines the rapid PINK1 turnover. PINK1 steady-state elimination by the N-end rule identifies a novel organelle to cytoplasm turnover pathway that yields a mechanism to flag damaged mitochondria for autophagic elimination.
Keywords: PARKIN; PARL; mitochondrial import; mitophagy; ubiquitin.
Figures


Similar articles
-
PHB2 (prohibitin 2) promotes PINK1-PRKN/Parkin-dependent mitophagy by the PARL-PGAM5-PINK1 axis.Autophagy. 2020 Mar;16(3):419-434. doi: 10.1080/15548627.2019.1628520. Epub 2019 Jun 16. Autophagy. 2020. PMID: 31177901 Free PMC article.
-
Intramembrane protease PARL defines a negative regulator of PINK1- and PARK2/Parkin-dependent mitophagy.Autophagy. 2015;11(9):1484-98. doi: 10.1080/15548627.2015.1063763. Autophagy. 2015. PMID: 26101826 Free PMC article.
-
N-degron-mediated degradation and regulation of mitochondrial PINK1 kinase.Curr Genet. 2020 Aug;66(4):693-701. doi: 10.1007/s00294-020-01062-2. Epub 2020 Mar 10. Curr Genet. 2020. PMID: 32157382 Review.
-
Functional alteration of PARL contributes to mitochondrial dysregulation in Parkinson's disease.Hum Mol Genet. 2011 May 15;20(10):1966-74. doi: 10.1093/hmg/ddr077. Epub 2011 Feb 25. Hum Mol Genet. 2011. PMID: 21355049
-
PINK1 import regulation at a crossroad of mitochondrial fate: the molecular mechanisms of PINK1 import.J Biochem. 2020 Mar 1;167(3):217-224. doi: 10.1093/jb/mvz069. J Biochem. 2020. PMID: 31504668 Review.
Cited by
-
Parkin, an E3 Ubiquitin Ligase, Plays an Essential Role in Mitochondrial Quality Control in Parkinson's Disease.Cell Mol Neurobiol. 2021 Oct;41(7):1395-1411. doi: 10.1007/s10571-020-00914-2. Epub 2020 Jul 4. Cell Mol Neurobiol. 2021. PMID: 32623547 Review.
-
Beyond mitophagy: cytosolic PINK1 as a messenger of mitochondrial health.Antioxid Redox Signal. 2015 Apr 20;22(12):1047-59. doi: 10.1089/ars.2014.6206. Epub 2015 Feb 18. Antioxid Redox Signal. 2015. PMID: 25557302 Free PMC article. Review.
-
Classical and Innovative Evidence for Therapeutic Strategies in Retinal Dysfunctions.Int J Mol Sci. 2024 Feb 9;25(4):2124. doi: 10.3390/ijms25042124. Int J Mol Sci. 2024. PMID: 38396799 Free PMC article. Review.
-
Mitochondrial Genome Variants as a Cause of Mitochondrial Cardiomyopathy.Cells. 2022 Sep 11;11(18):2835. doi: 10.3390/cells11182835. Cells. 2022. PMID: 36139411 Free PMC article. Review.
-
Heterozygous PINK1 p.G411S increases risk of Parkinson's disease via a dominant-negative mechanism.Brain. 2017 Jan;140(1):98-117. doi: 10.1093/brain/aww261. Epub 2016 Nov 2. Brain. 2017. PMID: 27807026 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
