Insights into the metabolic response to traumatic brain injury as revealed by (13)C NMR spectroscopy
- PMID: 24109452
- PMCID: PMC3790078
- DOI: 10.3389/fnene.2013.00008
Insights into the metabolic response to traumatic brain injury as revealed by (13)C NMR spectroscopy
Abstract
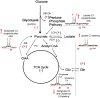
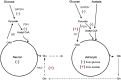
The present review highlights critical issues related to cerebral metabolism following traumatic brain injury (TBI) and the use of (13)C labeled substrates and nuclear magnetic resonance (NMR) spectroscopy to study these changes. First we address some pathophysiologic factors contributing to metabolic dysfunction following TBI. We then examine how (13)C NMR spectroscopy strategies have been used to investigate energy metabolism, neurotransmission, the intracellular redox state, and neuroglial compartmentation following injury. (13)C NMR spectroscopy studies of brain extracts from animal models of TBI have revealed enhanced glycolytic production of lactate, evidence of pentose phosphate pathway (PPP) activation, and alterations in neuronal and astrocyte oxidative metabolism that are dependent on injury severity. Differential incorporation of label into glutamate and glutamine from (13)C labeled glucose or acetate also suggest TBI-induced adaptations to the glutamate-glutamine cycle.
Keywords: acetate; glucose; glutamate-glutamine cycle; magnetic resonance spectroscopy; neuroglial compartmentation; oxidative metabolism; pentose phosphate pathway.
Figures
Similar articles
-
Metabolic fate of glucose in rats with traumatic brain injury and pyruvate or glucose treatments: A NMR spectroscopy study.Neurochem Int. 2017 Jan;102:66-78. doi: 10.1016/j.neuint.2016.11.014. Epub 2016 Dec 3. Neurochem Int. 2017. PMID: 27919624 Free PMC article.
-
Astrocyte oxidative metabolism and metabolite trafficking after fluid percussion brain injury in adult rats.J Neurotrauma. 2010 Dec;27(12):2191-202. doi: 10.1089/neu.2010.1508. Epub 2010 Nov 23. J Neurotrauma. 2010. PMID: 20939699 Free PMC article.
-
Glycolysis and the pentose phosphate pathway after human traumatic brain injury: microdialysis studies using 1,2-(13)C2 glucose.J Cereb Blood Flow Metab. 2015 Jan;35(1):111-20. doi: 10.1038/jcbfm.2014.177. Epub 2014 Oct 22. J Cereb Blood Flow Metab. 2015. PMID: 25335801 Free PMC article.
-
Translational Metabolomics of Head Injury: Exploring Dysfunctional Cerebral Metabolism with Ex Vivo NMR Spectroscopy-Based Metabolite Quantification.In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 25. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 25. PMID: 26269925 Free Books & Documents. Review.
-
Cerebral glucose metabolism and the glutamine cycle as detected by in vivo and in vitro 13C NMR spectroscopy.Neurochem Int. 2004 Jul-Aug;45(2-3):297-303. doi: 10.1016/j.neuint.2003.08.014. Neurochem Int. 2004. PMID: 15145545 Review.
Cited by
-
Brain temperature and its fundamental properties: a review for clinical neuroscientists.Front Neurosci. 2014 Oct 8;8:307. doi: 10.3389/fnins.2014.00307. eCollection 2014. Front Neurosci. 2014. PMID: 25339859 Free PMC article. Review.
-
Metabolic Alterations in Developing Brain After Injury: Knowns and Unknowns.Neurochem Res. 2015 Dec;40(12):2527-43. doi: 10.1007/s11064-015-1600-7. Epub 2015 Jul 7. Neurochem Res. 2015. PMID: 26148530 Free PMC article. Review.
-
The longitudinal biochemical profiling of TBI in a drop weight model of TBI.Sci Rep. 2023 Dec 14;13(1):22260. doi: 10.1038/s41598-023-48539-x. Sci Rep. 2023. PMID: 38097614 Free PMC article.
-
Carotid artery blood flow decreases after rapid head rotation in piglets.J Neurotrauma. 2015 Jan 15;32(2):120-6. doi: 10.1089/neu.2014.3570. Epub 2014 Nov 24. J Neurotrauma. 2015. PMID: 25133889 Free PMC article.
-
Plasma metabolomics profiles in rats with acute traumatic brain injury.PLoS One. 2017 Aug 3;12(8):e0182025. doi: 10.1371/journal.pone.0182025. eCollection 2017. PLoS One. 2017. PMID: 28771528 Free PMC article.
References
-
- Aureli T., Di Cocco M. E., Calvani M., Conti F. (1997). The entry of [1-13C] glucose into biochemical pathways reveals a complex compartmentation and metabolite trafficking between glia and neurons: a study by 13C-NMR spectroscopy. Brain Res. 765, 218–227 10.1016/S0006-8993(97)00514-3 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

