CD164 regulates the tumorigenesis of ovarian surface epithelial cells through the SDF-1α/CXCR4 axis
- PMID: 24094005
- PMCID: PMC4015273
- DOI: 10.1186/1476-4598-12-115
CD164 regulates the tumorigenesis of ovarian surface epithelial cells through the SDF-1α/CXCR4 axis
Abstract
Background: CD164 (endolyn), a sialomucin, has been reported to play a role in the proliferation, adhesion, and differentiation of hematopoietic stem cells. The potential association of CD164 with tumorigenicity remains unclear.
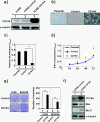
Methods: The clinicopathological correlation of ovarian cancer with CD164 was assessed in a 97-patient tumor tissue microarray. Overexpression or silence CD164 was to analyze the effect of CD164 on the proliferation, colony formation and apoptosis via a mouse xenograft and western blotting analysis. The subcellular localization of CD164 was collected in the immunohistochemical and confocal analysis.
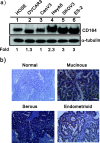
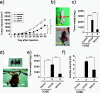
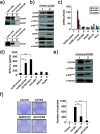
Results: Our data demonstrated that higher expression levels of CD164 were identified in malignant ovarian cancer cell lines, such as SKOV3 and HeyA8. The clinicopathological correlation analysis showed that the upregulation of CD164 protein was significantly associated with tumor grade and metastasis. The overexpression of CD164 in human ovarian epithelial surface cells promoted cellular proliferation and colony formation and suppressed apoptosis. These tumorigenicity effects of CD164 were reconfirmed in a mouse xenograft model. We also found that the overexpression of CD164 proteins increased the amounts of CXCR4 and SDF-1α and activated the SDF-1α/CXCR4 axis, inducing colony and sphere formation. Finally, we identified the subcellular localization of CD164 in the nucleus and cytosol and found that nuclear CD164 might be involved in the regulation of the activity of the CXCR4 promoter.
Conclusions: Our findings suggest that the increased expression of CD164 is involved in ovarian cancer progression via the SDF-1α/CXCR4 axis, which promotes tumorigenicity. Thus, targeting CD164 may serve as a potential ovarian cancer biomarker, and targeting CD164 may serve as a therapeutic modality in the management of high-grade ovarian tumors.
Figures



Similar articles
-
Involvement of SDF-1alpha/CXCR4 axis in the enhanced peritoneal metastasis of epithelial ovarian carcinoma.Int J Cancer. 2008 Jan 1;122(1):91-9. doi: 10.1002/ijc.23083. Int J Cancer. 2008. PMID: 17893878
-
[Relationship between chemokine axis CXCL12-CXCR4 and epithelial ovarian cancer].Zhonghua Yi Xue Za Zhi. 2013 Jun 4;93(21):1677-80. Zhonghua Yi Xue Za Zhi. 2013. PMID: 24125682 Chinese.
-
Cancer-associated fibroblasts promote the progression of endometrial cancer via the SDF-1/CXCR4 axis.J Hematol Oncol. 2016 Feb 6;9:8. doi: 10.1186/s13045-015-0231-4. J Hematol Oncol. 2016. PMID: 26851944 Free PMC article.
-
Intraoperative imaging in ovarian cancer: fact or fiction?Mol Imaging. 2011 Aug;10(4):248-57. doi: 10.2310/7290.2011.00004. Epub 2011 Apr 26. Mol Imaging. 2011. PMID: 21521557 Free PMC article. Review.
-
Animal models of ovarian cancer.Reprod Biol Endocrinol. 2003 Oct 7;1:67. doi: 10.1186/1477-7827-1-67. Reprod Biol Endocrinol. 2003. PMID: 14613552 Free PMC article. Review.
Cited by
-
Transcriptome profile of spleen tissues from locally-adapted Kenyan pigs (Sus scrofa) experimentally infected with three varying doses of a highly virulent African swine fever virus genotype IX isolate: Ken12/busia.1 (ken-1033).BMC Genomics. 2022 Jul 19;23(1):522. doi: 10.1186/s12864-022-08754-8. BMC Genomics. 2022. PMID: 35854219 Free PMC article.
-
In Vitro Characterization of the Human Skeletal Stem Cell-like Properties of Primary Bone-Derived Mesenchymal Stem/Stromal Cells in Patients with Late and Early Hip Osteoarthritis.Life (Basel). 2022 Jun 15;12(6):899. doi: 10.3390/life12060899. Life (Basel). 2022. PMID: 35743928 Free PMC article.
-
Knee and Peri-Knee Tissues of Post Mortem Donors Are Strategic Sources of Mesenchymal Stem/Stromal Cells for Regenerative Procedures.Int J Mol Sci. 2022 Mar 15;23(6):3170. doi: 10.3390/ijms23063170. Int J Mol Sci. 2022. PMID: 35328593 Free PMC article.
-
Alignment of single-cell trajectories by tuMap enables high-resolution quantitative comparison of cancer samples.Cell Syst. 2022 Jan 19;13(1):71-82.e8. doi: 10.1016/j.cels.2021.09.003. Epub 2021 Oct 7. Cell Syst. 2022. PMID: 34624253 Free PMC article.
-
RP11‑619L19.2 promotes colon cancer development by regulating the miR‑1271‑5p/CD164 axis.Oncol Rep. 2020 Dec;44(6):2419-2428. doi: 10.3892/or.2020.7794. Epub 2020 Oct 7. Oncol Rep. 2020. PMID: 33125110 Free PMC article.
References
-
- Naora H, Montell DJ. Ovarian cancer metastasis: integrating insights from disparate model organisms. Nat Rev Cancer. 2005;12:355–366. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

