Role of mitofusin 2 (Mfn2) in controlling cellular proliferation
- PMID: 24081906
- PMCID: PMC3868832
- DOI: 10.1096/fj.13-230037
Role of mitofusin 2 (Mfn2) in controlling cellular proliferation
Abstract
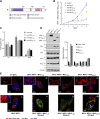
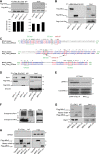
It has been reported that Mitofusin2 (Mfn2) inhibits cell proliferation when overexpressed. We wanted to study the role of endogenous Mfn2 in cell proliferation, along with the structural features of Mfn2 that influence its mitochondrial localization and control of cell proliferation. Mfn2-knockdown clones of a B-cell lymphoma cell line BJAB exhibited an increased rate of cell proliferation. A 2-fold increase in cell proliferation was also observed in Mfn2-knockout mouse embryonic fibroblast (MEF) cells as compared with the control wild-type cells, and the proliferative advantage of the knockout MEF cells was blocked on reintroduction of the Mfn2 gene. Mfn2 exerts its antiproliferative effect by acting as an effector molecule of Ras, resulting in the inhibition of the Ras-Raf-ERK signaling pathway. Furthermore, both the N-terminal (aa 1-264) and the C-terminal (aa 265-757) fragments of Mfn2 blocked cell proliferation through distinct mechanisms: the N-terminal-mediated inhibition was due to its interaction with Raf-1, whereas the C-terminal fragment of Mfn2 inhibited cell proliferation by interacting with Ras. The inhibition of proliferation by the N-terminal fragment was independent of its mitochondrial localization. Collectively, our data provide new insights regarding the role of Mfn2 in controlling cellular proliferation.
Keywords: ERK; HSG; Raf; Ras.
Figures
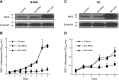
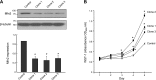
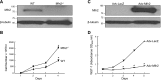
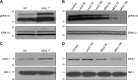


Similar articles
-
Mechanism of Activation-Induced Downregulation of Mitofusin 2 in Human Peripheral Blood T Cells.J Immunol. 2015 Dec 15;195(12):5780-6. doi: 10.4049/jimmunol.1501023. Epub 2015 Nov 13. J Immunol. 2015. PMID: 26566676 Free PMC article.
-
Mitofusion 2 Overexpression Decreased Proliferation of Human Embryonic Lung Fibroblasts in Acute Respiratory Distress Syndrome through Inhibiting RAS-RAF-1-ERK1/2 Pathway.Curr Med Sci. 2020 Dec;40(6):1092-1098. doi: 10.1007/s11596-020-2305-y. Epub 2021 Jan 11. Curr Med Sci. 2020. PMID: 33428137
-
Adiponectin affects vascular smooth muscle cell proliferation and apoptosis through modulation of the mitofusin-2-mediated Ras-Raf-Erk1/2 signaling pathway.Mol Med Rep. 2015 Sep;12(3):4703-4707. doi: 10.3892/mmr.2015.3899. Epub 2015 Jun 8. Mol Med Rep. 2015. PMID: 26059448
-
[Mitochondrial fusion protein Mfn2 and cardiovascular diseases].Sheng Li Ke Xue Jin Zhan. 2010 Feb;41(1):11-6. Sheng Li Ke Xue Jin Zhan. 2010. PMID: 21417008 Review. Chinese.
-
MFN2-related neuropathies: Clinical features, molecular pathogenesis and therapeutic perspectives.J Neurol Sci. 2015 Sep 15;356(1-2):7-18. doi: 10.1016/j.jns.2015.05.033. Epub 2015 May 29. J Neurol Sci. 2015. PMID: 26143526 Review.
Cited by
-
The role of mitochondrial dynamics in disease.MedComm (2020). 2023 Dec 28;4(6):e462. doi: 10.1002/mco2.462. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38156294 Free PMC article. Review.
-
PINK1-induced phosphorylation of mitofusin 2 at serine 442 causes its proteasomal degradation and promotes cell proliferation in lung cancer and pulmonary arterial hypertension.FASEB J. 2021 Aug;35(8):e21771. doi: 10.1096/fj.202100361R. FASEB J. 2021. PMID: 34275172 Free PMC article.
-
Mechanism of Activation-Induced Downregulation of Mitofusin 2 in Human Peripheral Blood T Cells.J Immunol. 2015 Dec 15;195(12):5780-6. doi: 10.4049/jimmunol.1501023. Epub 2015 Nov 13. J Immunol. 2015. PMID: 26566676 Free PMC article.
-
Metabolic Stress-Induced Phosphorylation of KAP1 Ser473 Blocks Mitochondrial Fusion in Breast Cancer Cells.Cancer Res. 2016 Sep 1;76(17):5006-18. doi: 10.1158/0008-5472.CAN-15-2921. Epub 2016 Jun 30. Cancer Res. 2016. PMID: 27364555 Free PMC article.
-
Activation of Mitofusin2 by Smad2-RIN1 Complex during Mitochondrial Fusion.Mol Cell. 2016 May 19;62(4):520-31. doi: 10.1016/j.molcel.2016.04.010. Epub 2016 May 12. Mol Cell. 2016. PMID: 27184078 Free PMC article.
References
-
- De Brito O. M., Scorrano L. (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456, 605–610 - PubMed
-
- De Brito O. M., Scorrano L. (2009) Mitofusin-2 regulates mitochondrial and endoplasmic reticulum morphology and tethering: the role of Ras. Mitochondrion 9, 222–226 - PubMed
-
- Zuchner S., Mersiyanova I. V., Muglia M., Bissar-Tadmouri N., Rochelle J., Dadali E. L., Zappia M., Nelis E., Patitucci A., Senderek J., Parman Y., Evgrafov O., Jonghe P. D., Takahashi Y., Tsuji S., Pericak-Vance M. A., Quattrone A., Battaloglu E., Polyakov A. V., Timmerman V., Schroder J. M., Vance J. M. (2004) Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat. Genet. 36, 449–451 - PubMed
-
- Hernandez-Alvarez M. I., Thabit H., Burns N., Shah S., Brema I., Hatunic M., Finucane F., Liesa M., Chiellini C., Naon D., Zorzano A., Nolan J. J. (2010) Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1α/mitofusin-2 regulatory pathway in response to physical activity. Diabetes Care 33, 645–651 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

