A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults
- PMID: 24080653
- PMCID: PMC3837853
- DOI: 10.1128/AAC.01285-13
A novel investigational Fc-modified humanized monoclonal antibody, motavizumab-YTE, has an extended half-life in healthy adults
Abstract
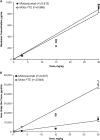
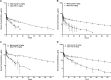
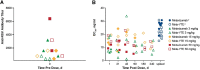
The study objective was to evaluate the pharmacokinetics (PK), antidrug antibody (ADA), and safety of motavizumab-YTE (motavizumab with amino acid substitutions M252Y/S254T/T256E [YTE]), an Fc-modified anti-respiratory syncytial virus (RSV) monoclonal antibody. Healthy adults (n = 31) were randomized to receive a single intravenous (i.v.) dose of motavizumab-YTE or motavizumab (0.3, 3, 15, or 30 mg/kg) and followed for 240 days. Clearance of motavizumab-YTE was significantly lower (71% to 86%) and the half-life (t1/2) was 2- to 4-fold longer than with motavizumab. However, similar peak concentrations and volume-of-distribution values, indicative of similar distribution properties, were seen at all dose levels. The sustained serum concentrations of motavizumab-YTE were fully functional, as shown by RSV neutralizing activity that persisted for 240 days with motavizumab-YTE versus 90 days postdose for motavizumab. Safety and incidence of ADA were comparable between groups. In this first study of an Fc-modified monoclonal antibody in humans, motavizumab-YTE was well tolerated and exhibited an extended half-life of up to 100 days. (This study has been registered at ClinicalTrials.gov under registration no. NCT00578682.).
Figures
Similar articles
-
A phase 2, randomized, double-blind safety and pharmacokinetic assessment of respiratory syncytial virus (RSV) prophylaxis with motavizumab and palivizumab administered in the same season.BMC Pediatr. 2010 Jun 3;10:38. doi: 10.1186/1471-2431-10-38. BMC Pediatr. 2010. PMID: 20525274 Free PMC article. Clinical Trial.
-
Safety, Tolerability, and Pharmacokinetics of MEDI4893, an Investigational, Extended-Half-Life, Anti-Staphylococcus aureus Alpha-Toxin Human Monoclonal Antibody, in Healthy Adults.Antimicrob Agents Chemother. 2016 Dec 27;61(1):e01020-16. doi: 10.1128/AAC.01020-16. Print 2017 Jan. Antimicrob Agents Chemother. 2016. PMID: 27795368 Free PMC article. Clinical Trial.
-
Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn).J Biol Chem. 2006 Aug 18;281(33):23514-24. doi: 10.1074/jbc.M604292200. Epub 2006 Jun 21. J Biol Chem. 2006. PMID: 16793771
-
Immunoprophylaxis of RSV infection: advancing from RSV-IGIV to palivizumab and motavizumab.Curr Top Microbiol Immunol. 2008;317:103-23. doi: 10.1007/978-3-540-72146-8_4. Curr Top Microbiol Immunol. 2008. PMID: 17990791 Review.
-
Monoclonal antibody for reducing the risk of respiratory syncytial virus infection in children.Cochrane Database Syst Rev. 2013 Apr 30;(4):CD006602. doi: 10.1002/14651858.CD006602.pub4. Cochrane Database Syst Rev. 2013. PMID: 23633336 Review.
Cited by
-
Impact of structural modifications of IgG antibodies on effector functions.Front Immunol. 2024 Jan 8;14:1304365. doi: 10.3389/fimmu.2023.1304365. eCollection 2023. Front Immunol. 2024. PMID: 38259472 Free PMC article. Review.
-
Preclinical and Clinical Investigations of Potential Drugs and Vaccines for COVID-19 Therapy: A Comprehensive Review With Recent Update.Clin Pathol. 2024 Jul 26;17:2632010X241263054. doi: 10.1177/2632010X241263054. eCollection 2024 Jan-Dec. Clin Pathol. 2024. PMID: 39070952 Free PMC article. Review.
-
Mechanistic incorporation of FcRn binding in plasma and endosomes in a whole body PBPK model for large molecules.J Pharmacokinet Pharmacodyn. 2023 Jun;50(3):229-241. doi: 10.1007/s10928-023-09849-9. Epub 2023 Mar 6. J Pharmacokinet Pharmacodyn. 2023. PMID: 36877385
-
Prevention of Pediatric Respiratory Syncytial Virus Lower Respiratory Tract Illness: Perspectives for the Next Decade.Front Immunol. 2019 May 7;10:1006. doi: 10.3389/fimmu.2019.01006. eCollection 2019. Front Immunol. 2019. PMID: 31134078 Free PMC article. Review.
-
A safe and efficient BCG vectored vaccine to prevent the disease caused by the human Respiratory Syncytial Virus.Hum Vaccin Immunother. 2017 Sep 2;13(9):2092-2097. doi: 10.1080/21645515.2017.1334026. Epub 2017 Jun 9. Hum Vaccin Immunother. 2017. PMID: 28598702 Free PMC article. Review.
References
-
- American Academy of Pediatrics 2012. Respiratory syncytial virus, p 609–617 In Pickering LK, Baker CJ, Kimberlin DW, Long SS. (ed), Red book: 2012 report of the Committee on Infectious Diseases, 29th ed. American Academy of Pediatrics, Elk Grove Village, IL
-
- Mould DR, Sweeney KR. 2007. The pharmacokinetics and pharmacodynamics of monoclonal antibodies—mechanistic modeling applied to drug development. Curr. Opin. Drug Discov. Devel. 10:84–96 - PubMed
-
- Roopenian DC, Akilesh S. 2007. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7:715–725 - PubMed
-
- Simister NE, Mostov KE. 1989. An Fc receptor structurally related to MHC class I antigens. Nature 337:184–187 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

