A mechanistic basis for the co-evolution of chicken tapasin and major histocompatibility complex class I (MHC I) proteins
- PMID: 24078633
- PMCID: PMC3820913
- DOI: 10.1074/jbc.M113.474031
A mechanistic basis for the co-evolution of chicken tapasin and major histocompatibility complex class I (MHC I) proteins
Abstract
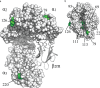
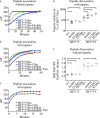
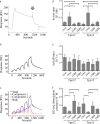
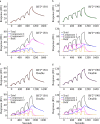
MHC class I molecules display peptides at the cell surface to cytotoxic T cells. The co-factor tapasin functions to ensure that MHC I becomes loaded with high affinity peptides. In most mammals, the tapasin gene appears to have little sequence diversity and few alleles and is located distal to several classical MHC I loci, so tapasin appears to function in a universal way to assist MHC I peptide loading. In contrast, the chicken tapasin gene is tightly linked to the single dominantly expressed MHC I locus and is highly polymorphic and moderately diverse in sequence. Therefore, tapasin-assisted loading of MHC I in chickens may occur in a haplotype-specific way, via the co-evolution of chicken tapasin and MHC I. Here we demonstrate a mechanistic basis for this co-evolution, revealing differences in the ability of two chicken MHC I alleles to bind and release peptides in the presence or absence of tapasin, where, as in mammals, efficient self-loading is negatively correlated with tapasin-assisted loading. We found that a polymorphic residue in the MHC I α3 domain thought to bind tapasin influenced both tapasin function and intrinsic peptide binding properties. Differences were also evident between the MHC alleles in their interactions with tapasin. Last, we show that a mismatched combination of tapasin and MHC alleles exhibit significantly impaired MHC I maturation in vivo and that polymorphic MHC residues thought to contact tapasin influence maturation efficiency. Collectively, this supports the possibility that tapasin and BF2 proteins have co-evolved, resulting in allele-specific peptide loading in vivo.
Keywords: Antigen Presentation; Chicken MHC; Co-evolution; Evolution; Immunology; Major Histocompatibility Complex (MHC); Peptide Selection; Protein-Protein Interactions; Tapasin.
Figures


Similar articles
-
Two polymorphisms facilitate differences in plasticity between two chicken major histocompatibility complex class I proteins.PLoS One. 2014 Feb 20;9(2):e89657. doi: 10.1371/journal.pone.0089657. eCollection 2014. PLoS One. 2014. PMID: 24586943 Free PMC article.
-
Plasticity of empty major histocompatibility complex class I molecules determines peptide-selector function.Mol Immunol. 2015 Dec;68(2 Pt A):98-101. doi: 10.1016/j.molimm.2015.03.010. Epub 2015 Mar 26. Mol Immunol. 2015. PMID: 25818313 Free PMC article. Review.
-
Molecular architecture of the MHC I peptide-loading complex: one tapasin molecule is essential and sufficient for antigen processing.FASEB J. 2012 Dec;26(12):5071-80. doi: 10.1096/fj.12-217489. Epub 2012 Aug 24. FASEB J. 2012. PMID: 22923333
-
A single polymorphic residue within the peptide-binding cleft of MHC class I molecules determines spectrum of tapasin dependence.J Immunol. 2003 Jan 15;170(2):961-8. doi: 10.4049/jimmunol.170.2.961. J Immunol. 2003. PMID: 12517962
-
Antigen processing and presentation: evolution from a bird's eye view.Mol Immunol. 2013 Sep;55(2):159-61. doi: 10.1016/j.molimm.2012.10.030. Epub 2012 Nov 22. Mol Immunol. 2013. PMID: 23182425 Free PMC article. Review.
Cited by
-
Generalists and Specialists: A New View of How MHC Class I Molecules Fight Infectious Pathogens.Trends Immunol. 2018 May;39(5):367-379. doi: 10.1016/j.it.2018.01.001. Epub 2018 Jan 31. Trends Immunol. 2018. PMID: 29396014 Free PMC article. Review.
-
Selector function of MHC I molecules is determined by protein plasticity.Sci Rep. 2015 Oct 20;5:14928. doi: 10.1038/srep14928. Sci Rep. 2015. PMID: 26482009 Free PMC article.
-
Direct evidence for conformational dynamics in major histocompatibility complex class I molecules.J Biol Chem. 2017 Dec 8;292(49):20255-20269. doi: 10.1074/jbc.M117.809624. Epub 2017 Oct 11. J Biol Chem. 2017. PMID: 29021251 Free PMC article.
-
Chickens as a simple system for scientific discovery: The example of the MHC.Mol Immunol. 2021 Jul;135:12-20. doi: 10.1016/j.molimm.2021.03.019. Epub 2021 Apr 9. Mol Immunol. 2021. PMID: 33845329 Free PMC article.
-
Molecular mechanism of peptide editing in the tapasin-MHC I complex.Sci Rep. 2016 Jan 12;6:19085. doi: 10.1038/srep19085. Sci Rep. 2016. PMID: 26754481 Free PMC article.
References
-
- Sadasivan B., Lehner P. J., Ortmann B., Spies T., Cresswell P. (1996) Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity 5, 103–114 - PubMed
-
- Dick T. P., Bangia N., Peaper D. R., Cresswell P. (2002) Disulfide bond isomerization and the assembly of MHC class I-peptide complexes. Immunity 16, 87–98 - PubMed
-
- Van Hateren A., James E., Bailey A., Phillips A., Dalchau N., Elliott T. (2010) The cell biology of major histocompatibility complex class I assembly. Towards a molecular understanding. Tissue Antigens 76, 259–275 - PubMed
-
- Lewis J. W., Elliott T. (1998) Evidence for successive peptide binding and quality control stages during MHC class I assembly. Curr. Biol. 8, 717–720 - PubMed
-
- Williams A. P., Peh C. A., Purcell A. W., McCluskey J., Elliott T. (2002) Optimization of the MHC class I peptide cargo is dependent on tapasin. Immunity 16, 509–520 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

