Glucosamine hydrochloride exerts a protective effect against unilateral ureteral obstruction-induced renal fibrosis by attenuating TGF-β signaling
- PMID: 24072041
- PMCID: PMC3825548
- DOI: 10.1007/s00109-013-1086-1
Glucosamine hydrochloride exerts a protective effect against unilateral ureteral obstruction-induced renal fibrosis by attenuating TGF-β signaling
Abstract
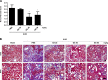
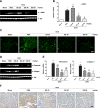
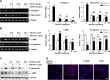
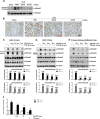
Renal fibrosis is a common consequence of unilateral ureteral obstruction, which provides a useful model to investigate the pathogenesis of obstructive nephropathy and progressive renal fibrosis. Transforming growth factor (TGF-β1) has been recognized as a key mediator in renal fibrosis by stimulating matrix-producing fibrogenic cells and promoting extracellular matrix deposition. Therefore, considerable efforts have been made to regulate TGF-β signaling for antifibrotic therapy. Here, we investigated the mode of action of glucosamine hydrochloride (GS-HCl) on TGF-β1-induced renal fibrosis. In the obstructed kidneys and TGF-β1-treated renal cells, GS-HCl significantly decreased renal expression of α-smooth muscle actin, collagen I, and fibronectin. By investigating the inhibitory mechanism of GS-HCl on renal fibrosis, we found that GS-HCl suppressed TGF-β signaling by inhibiting N-linked glycosylation of the type II TGF-β receptor (TβRII), leading to an inefficient trafficking of TβRII to the membrane surface. Defective N-glycosylation of TβRII further suppressed the TGF-β1-binding to TβRII, thereby decreasing TGF-β signaling. Notably, GS-HCl treatment significantly reduced TGF-β1-induced up-regulation of Smad2/3 phosphorylation and transcriptional activity in vivo and in vitro. Taken together, GS-HCl-mediated regulation of TGF-β signaling exerted an antifibrotic effect, thereby ameliorating renal fibrosis. Our study suggests that GS-HCl would be a promising agent for therapeutic intervention for preventing TGF-β1-induced renal fibrosis in kidney diseases.
Key message: Glucosamine-mediated attenuation of TGF-β signaling ameliorates renal fibrosis in vivo TGF-β1-induced fibrogenic action is reduced by glucosamine in vitro N-glycosylation of the type II TGF-β receptor is suppressed by glucosamine Glucosamine-induced defective N-glycosylation of TβRII decreases TGF-β signaling.
Figures

Similar articles
-
HuangQi Decoction Ameliorates Renal Fibrosis via TGF-β/Smad Signaling Pathway In Vivo and In Vitro.Cell Physiol Biochem. 2016;38(5):1761-74. doi: 10.1159/000443115. Epub 2016 May 9. Cell Physiol Biochem. 2016. PMID: 27161221
-
Apamin inhibits renal fibrosis via suppressing TGF-β1 and STAT3 signaling in vivo and in vitro.J Mol Med (Berl). 2021 Sep;99(9):1265-1277. doi: 10.1007/s00109-021-02087-x. Epub 2021 May 24. J Mol Med (Berl). 2021. PMID: 34031696
-
Heat shock protein 90 inhibitor attenuates renal fibrosis through degradation of transforming growth factor-β type II receptor.Lab Invest. 2012 Nov;92(11):1583-96. doi: 10.1038/labinvest.2012.127. Epub 2012 Sep 10. Lab Invest. 2012. PMID: 22964853
-
TGF-β1 → SMAD/p53/USF2 → PAI-1 transcriptional axis in ureteral obstruction-induced renal fibrosis.Cell Tissue Res. 2012 Jan;347(1):117-28. doi: 10.1007/s00441-011-1181-y. Epub 2011 Jun 4. Cell Tissue Res. 2012. PMID: 21638209 Free PMC article. Review.
-
Mutual regulation between glycosylation and transforming growth factor-β isoforms signaling pathway.Int J Biol Macromol. 2023 May 1;236:123818. doi: 10.1016/j.ijbiomac.2023.123818. Epub 2023 Feb 27. Int J Biol Macromol. 2023. PMID: 36858092 Review.
Cited by
-
Abelmoschus esculentus subfractions improved nephropathy with regulating dipeptidyl peptidase-4 and type 1 glucagon-like peptide receptor in type 2 diabetic rats.J Food Drug Anal. 2019 Jan;27(1):135-144. doi: 10.1016/j.jfda.2018.07.004. Epub 2018 Aug 14. J Food Drug Anal. 2019. PMID: 30648566 Free PMC article.
-
Glycosylation in health and disease.Nat Rev Nephrol. 2019 Jun;15(6):346-366. doi: 10.1038/s41581-019-0129-4. Nat Rev Nephrol. 2019. PMID: 30858582 Free PMC article. Review.
-
Glucosamine inhibits extracellular matrix accumulation in experimental diabetic nephropathy.Front Nutr. 2022 Dec 1;9:1048305. doi: 10.3389/fnut.2022.1048305. eCollection 2022. Front Nutr. 2022. PMID: 36532524 Free PMC article.
-
Glucosamine inhibits IL-1β expression by preserving mitochondrial integrity and disrupting assembly of the NLRP3 inflammasome.Sci Rep. 2019 Apr 3;9(1):5603. doi: 10.1038/s41598-019-42130-z. Sci Rep. 2019. PMID: 30944389 Free PMC article.
-
Rapid recovery of male cats with postrenal acute kidney injury by treating with allogeneic adipose mesenchymal stem cell-derived extracellular vesicles.Stem Cell Res Ther. 2022 Jul 28;13(1):379. doi: 10.1186/s13287-022-03039-z. Stem Cell Res Ther. 2022. PMID: 35902973 Free PMC article.
References
-
- Anderson JW, Nicolosi RJ, Borzelleca JF (2005) Glucosamine effects in humans: a review of effects on glucose metabolism, side effects, safety considerations and efficacy. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association 43:187–201. DOI 10.1016/j.fct.2004.11.006 - PubMed
-
- Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Bruyere O, Giacovelli G, Henrotin Y, Dacre JE, Gossett C. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357:251–256. doi: 10.1016/S0140-6736(00)03610-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

