Species differences and molecular determinant of TRPA1 cold sensitivity
- PMID: 24071625
- PMCID: PMC3791479
- DOI: 10.1038/ncomms3501
Species differences and molecular determinant of TRPA1 cold sensitivity
Abstract
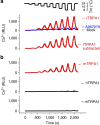
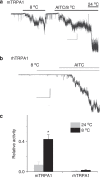
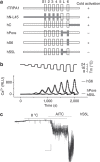
TRPA1 is an ion channel and has been proposed as a thermosensor across species. In invertebrate and ancestral vertebrates such as fly, mosquito, frog, lizard and snakes, TRPA1 serves as a heat receptor, a sensory input utilized for heat avoidance or infrared detection. However, in mammals, whether TRPA1 is a receptor for noxious cold is highly controversial, as channel activation by cold was observed by some groups but disputed by others. Here we attribute the discrepancy to species differences. We show that cold activates rat and mouse TRPA1 but not human or rhesus monkey TRPA1. At the molecular level, a single residue within the S5 transmembrane domain (G878 in rodent but V875 in primate) accounts for the observed difference in cold sensitivity. This residue difference also underlies the species-specific effects of menthol. Together, our findings identify the species-specific cold activation of TRPA1 and reveal a molecular determinant of cold-sensitive gating.
Conflict of interest statement
J.C., J.X., M.L., C.S., K.W. and B.Y. are AbbVie employees. All other authors declare no competing financial interests.
Figures



Similar articles
-
Structural modeling and patch-clamp analysis of pain-related mutation TRPA1-N855S reveal inter-subunit salt bridges stabilizing the channel open state.Neuropharmacology. 2015 Jun;93:294-307. doi: 10.1016/j.neuropharm.2015.02.018. Epub 2015 Feb 25. Neuropharmacology. 2015. PMID: 25724085
-
The molecular basis for species-specific activation of human TRPA1 protein by protons involves poorly conserved residues within transmembrane domains 5 and 6.J Biol Chem. 2013 Jul 12;288(28):20280-92. doi: 10.1074/jbc.M113.479337. Epub 2013 May 24. J Biol Chem. 2013. PMID: 23709225 Free PMC article.
-
Molecular determinants of species-specific activation or blockade of TRPA1 channels.J Neurosci. 2008 May 7;28(19):5063-71. doi: 10.1523/JNEUROSCI.0047-08.2008. J Neurosci. 2008. PMID: 18463259 Free PMC article.
-
Irritating channels: the case of TRPA1.J Physiol. 2011 Apr 1;589(Pt 7):1543-9. doi: 10.1113/jphysiol.2010.200717. Epub 2010 Nov 15. J Physiol. 2011. PMID: 21078588 Free PMC article. Review.
-
TRPA1 and cold transduction: an unresolved issue?J Gen Physiol. 2009 Mar;133(3):245-9. doi: 10.1085/jgp.200810136. J Gen Physiol. 2009. PMID: 19237589 Free PMC article. Review. No abstract available.
Cited by
-
Structural and in Vitro Functional Characterization of a Menthyl TRPM8 Antagonist Indicates Species-Dependent Regulation.ACS Med Chem Lett. 2021 Mar 31;12(5):758-767. doi: 10.1021/acsmedchemlett.1c00001. eCollection 2021 May 13. ACS Med Chem Lett. 2021. PMID: 34055223 Free PMC article.
-
Identification of transient receptor potential channel genes from the swimming crab, Portunus Trituberculatus, and their expression profiles under acute temperature stress.BMC Genomics. 2024 Jan 17;25(1):72. doi: 10.1186/s12864-024-09973-x. BMC Genomics. 2024. PMID: 38233779 Free PMC article.
-
Phosphoinositide-interacting regulator of TRP (PIRT) has opposing effects on human and mouse TRPM8 ion channels.J Biol Chem. 2018 Jun 15;293(24):9423-9434. doi: 10.1074/jbc.RA118.003563. Epub 2018 May 3. J Biol Chem. 2018. PMID: 29724821 Free PMC article.
-
Nociceptor Signalling through ion Channel Regulation via GPCRs.Int J Mol Sci. 2019 May 20;20(10):2488. doi: 10.3390/ijms20102488. Int J Mol Sci. 2019. PMID: 31137507 Free PMC article. Review.
-
Do free-ranging rattlesnakes use thermal cues to evaluate prey?J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2018 Mar;204(3):295-303. doi: 10.1007/s00359-017-1239-8. Epub 2017 Dec 7. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2018. PMID: 29218413
References
-
- Bandell M. et al.. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41, 849–857 (2004). - PubMed
-
- Bautista D. M. et al.. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282 (2006). - PubMed
-
- Jordt S. E. et al.. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427, 260–265 (2004). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

