Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control
- PMID: 24067431
- PMCID: PMC3973038
- DOI: 10.1038/ki.2013.356
Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control
Abstract
In patients with diabetes, glycemic improvement by sodium-glucose cotransporter-2 inhibition depends on the kidney's ability to filter glucose. Dapagliflozin, a sodium-glucose cotransporter-2 inhibitor, reduces hyperglycemia in patients with diabetes and normal or mildly impaired renal function. In this randomized, double-blind, placebo-controlled study we assessed daily treatment with dapagliflozin in 252 patients with inadequately controlled type 2 diabetes and moderate renal impairment. The primary endpoint, the mean change in HbA1c, was not statistically different from placebo after 24 weeks (-0.41% and -0.44% for 5- and 10-mg doses, respectively, and -0.32% for placebo). The mean weight change from baseline was -1.54 and -1.89 kg for the 5- and 10-mg doses, respectively, and +0.21 kg for placebo. The mean systolic and diastolic blood pressure decreased in the dapagliflozin groups compared to placebo. Through 104 weeks, 13 patients receiving dapagliflozin and no patients receiving placebo experienced bone fracture. At 1 week, the mean serum creatinine increased with dapagliflozin 5 mg (+0.13 mg/dl) and 10 mg (+0.18 mg/dl) and did not change further after 104 weeks. Mean serum electrolytes did not change in any group, and there were fewer episodes of hyperkalemia with dapagliflozin than placebo. Thus, in patients with moderate renal impairment, dapagliflozin did not improve glycemic control, but reduced weight and blood pressure.
Trial registration: ClinicalTrials.gov NCT00663260.
Figures

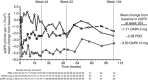
Comment in
-
Diabetes: efficacy of dapagliflozin associated with renal function.Nat Rev Nephrol. 2013 Dec;9(12):695. doi: 10.1038/nrneph.2013.216. Epub 2013 Oct 15. Nat Rev Nephrol. 2013. PMID: 24126593 No abstract available.
-
The perils of clinical trials.Kidney Int. 2014 Apr;85(4):745-7. doi: 10.1038/ki.2013.406. Kidney Int. 2014. PMID: 24682123
-
Dapagliflozin does not show improvement in glycemic control in moderate renal impairment: a question of analytical rigor?Kidney Int. 2014 Dec;86(6):1271-2. doi: 10.1038/ki.2014.290. Kidney Int. 2014. PMID: 25427091 No abstract available.
-
The authors reply.Kidney Int. 2014 Dec;86(6):1272. doi: 10.1038/ki.2014.291. Kidney Int. 2014. PMID: 25427094 No abstract available.
Similar articles
-
[Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin].Dtsch Med Wochenschr. 2013 Apr;138 Suppl 1:S27-38. doi: 10.1055/s-0032-1305284. Epub 2013 Mar 25. Dtsch Med Wochenschr. 2013. PMID: 23529568 Clinical Trial. German.
-
Albuminuria-lowering effect of dapagliflozin alone and in combination with saxagliptin and effect of dapagliflozin and saxagliptin on glycaemic control in patients with type 2 diabetes and chronic kidney disease (DELIGHT): a randomised, double-blind, placebo-controlled trial.Lancet Diabetes Endocrinol. 2019 Jun;7(6):429-441. doi: 10.1016/S2213-8587(19)30086-5. Epub 2019 Apr 13. Lancet Diabetes Endocrinol. 2019. PMID: 30992195 Clinical Trial.
-
Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double-blind, placebo-controlled, phase 3 study.Lancet Diabetes Endocrinol. 2016 Mar;4(3):211-220. doi: 10.1016/S2213-8587(15)00417-9. Epub 2015 Nov 27. Lancet Diabetes Endocrinol. 2016. PMID: 26620248 Clinical Trial.
-
Efficacy and safety of dapagliflozin in Asian patients with type 2 diabetes after metformin failure: A randomized controlled trial.J Diabetes. 2016 Nov;8(6):796-808. doi: 10.1111/1753-0407.12357. Epub 2016 Jan 9. J Diabetes. 2016. PMID: 26589253 Clinical Trial.
-
Dapagliflozin: a new sodium-glucose cotransporter 2 inhibitor for treatment of type 2 diabetes.Am J Health Syst Pharm. 2015 Mar 1;72(5):361-72. doi: 10.2146/ajhp140168. Am J Health Syst Pharm. 2015. PMID: 25694411 Review.
Cited by
-
SGLT2 Inhibitors: Benefit/Risk Balance.Curr Diab Rep. 2016 Oct;16(10):92. doi: 10.1007/s11892-016-0789-4. Curr Diab Rep. 2016. PMID: 27541294 Review.
-
Dapagliflozin reverses the imbalance of T helper 17 and T regulatory cells by inhibiting SGK1 in a mouse model of diabetic kidney disease.FEBS Open Bio. 2021 May;11(5):1395-1405. doi: 10.1002/2211-5463.13147. Epub 2021 May 1. FEBS Open Bio. 2021. PMID: 33728820 Free PMC article.
-
Glucuretic effects and renal safety of dapagliflozin in patients with type 2 diabetes.Ther Adv Endocrinol Metab. 2015 Jun;6(3):92-102. doi: 10.1177/2042018815575273. Ther Adv Endocrinol Metab. 2015. PMID: 26137213 Free PMC article. Review.
-
Role of Gut Microbiota, Immune Imbalance, and Allostatic Load in the Occurrence and Development of Diabetic Kidney Disease.J Diabetes Res. 2023 Dec 6;2023:8871677. doi: 10.1155/2023/8871677. eCollection 2023. J Diabetes Res. 2023. PMID: 38094870 Free PMC article. Review.
-
Links between Metabolic Syndrome and Hypertension: The Relationship with the Current Antidiabetic Drugs.Metabolites. 2023 Jan 5;13(1):87. doi: 10.3390/metabo13010087. Metabolites. 2023. PMID: 36677012 Free PMC article. Review.
References
-
- Tahrani AA, Bailey CJ, Del Prato S, et al. Management of type 2 diabetes: new and future developments in treatment. Lancet. 2011;378:182–197. - PubMed
-
- Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr. 2003;89:3–9. - PubMed
-
- Bailey CJ, Gross JL, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;375:2223–2233. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

