Preclinical development of novel Rac1-GEF signaling inhibitors using a rational design approach in highly aggressive breast cancer cell lines
- PMID: 24066799
- PMCID: PMC4104455
- DOI: 10.2174/18715206113136660334
Preclinical development of novel Rac1-GEF signaling inhibitors using a rational design approach in highly aggressive breast cancer cell lines
Abstract
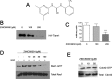
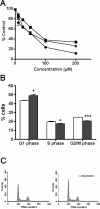
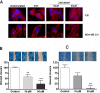
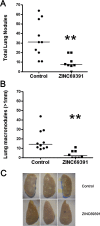
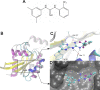
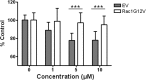
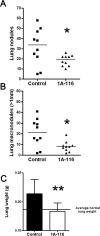
Rho GTPases play a key role in the regulation of multiple essential cellular processes, including actin dynamics, gene transcription and cell cycle progression. Aberrant activation of Rac1, a member of Rho family of small GTPases, is associated with tumorigenesis, cancer progression, invasion and metastasis. Particularly, Rac1 is overexpressed and hyperactivated in highly aggressive breast cancer. Thus, Rac1 appears to be a promising and relevant target for the development of novel anticancer drugs. We identified the novel Rac1 inhibitor ZINC69391 through a docking-based virtual library screening targeting Rac1 activation by GEFs. This compound was able to block Rac1 interaction with its GEF Tiam1, prevented EGF-induced Rac1 activation and inhibited cell proliferation, cell migration and cell cycle progression in highly aggressive breast cancer cell lines. Moreover, ZINC69391 showed an in vivo antimetastatic effect in a syngeneic animal model. We further developed the novel analog 1A-116 by rational design and showed to be specific and more potent than the parental compound in vitro and interfered Rac1-P-Rex1 interaction. We also showed an enhanced in vivo potency of 1A-116 analog. These results show that we have developed novel Rac1 inhibitors that may be used as a novel anticancer therapy.
Figures

Similar articles
-
Development of an Improved Guanidine-Based Rac1 Inhibitor with in vivo Activity against Non-Small Cell Lung Cancer.ChemMedChem. 2021 Mar 18;16(6):1011-1021. doi: 10.1002/cmdc.202000763. Epub 2021 Jan 25. ChemMedChem. 2021. PMID: 33284505
-
The guanine nucleotide exchange factor Tiam1: a Janus-faced molecule in cellular signaling.Cell Signal. 2014 Mar;26(3):483-91. doi: 10.1016/j.cellsig.2013.11.034. Epub 2013 Dec 2. Cell Signal. 2014. PMID: 24308970 Review.
-
A Rac1/Cdc42 GTPase-specific small molecule inhibitor suppresses growth of primary human prostate cancer xenografts and prolongs survival in mice.PLoS One. 2013 Sep 11;8(9):e74924. doi: 10.1371/journal.pone.0074924. eCollection 2013. PLoS One. 2013. PMID: 24040362 Free PMC article.
-
Cucurbitacin I inhibits Rac1 activation in breast cancer cells by a reactive oxygen species-mediated mechanism and independently of Janus tyrosine kinase 2 and P-Rex1.Mol Pharmacol. 2013 May;83(5):1141-54. doi: 10.1124/mol.112.084293. Epub 2013 Mar 11. Mol Pharmacol. 2013. PMID: 23478800 Free PMC article.
-
GEFs: Dual regulation of Rac1 signaling.Small GTPases. 2017 Apr 3;8(2):90-99. doi: 10.1080/21541248.2016.1202635. Epub 2016 Jun 17. Small GTPases. 2017. PMID: 27314616 Free PMC article. Review.
Cited by
-
Preclinical Efficacy and Toxicology Evaluation of RAC1 Inhibitor 1A-116 in Human Glioblastoma Models.Cancers (Basel). 2022 Sep 30;14(19):4810. doi: 10.3390/cancers14194810. Cancers (Basel). 2022. PMID: 36230732 Free PMC article.
-
Role of Host Small GTPases in Apicomplexan Parasite Infection.Microorganisms. 2022 Jul 7;10(7):1370. doi: 10.3390/microorganisms10071370. Microorganisms. 2022. PMID: 35889089 Free PMC article. Review.
-
Targeting Small GTPases and Their Prenylation in Diabetes Mellitus.J Med Chem. 2021 Jul 22;64(14):9677-9710. doi: 10.1021/acs.jmedchem.1c00410. Epub 2021 Jul 8. J Med Chem. 2021. PMID: 34236862 Free PMC article. Review.
-
Homology Model and Docking-Based Virtual Screening for Ligands of Human Dyskerin as New Inhibitors of Telomerase for Cancer Treatment.Int J Mol Sci. 2018 Oct 18;19(10):3216. doi: 10.3390/ijms19103216. Int J Mol Sci. 2018. PMID: 30340325 Free PMC article.
-
Stress-Sensitive Protein Rac1 and Its Involvement in Neurodevelopmental Disorders.Neural Plast. 2020 Nov 24;2020:8894372. doi: 10.1155/2020/8894372. eCollection 2020. Neural Plast. 2020. PMID: 33299404 Free PMC article. Review.
References
-
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–635. - PubMed
-
- Ellenbroek SI, Collard JG. Rho GTPases: Functions and association with cancer. Clin. Exp. Metastasis. 2007;24(8):657–672. - PubMed
-
- Fritz G, Just I, Kaina B. Rho GTPases are over-expressed in human tumors. Int. J. Cancer. 1999;81(5):682–687. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
