Diet-induced obesity suppresses expression of many proteins at the blood-brain barrier
- PMID: 24064496
- PMCID: PMC3887347
- DOI: 10.1038/jcbfm.2013.166
Diet-induced obesity suppresses expression of many proteins at the blood-brain barrier
Abstract
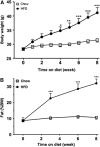
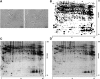
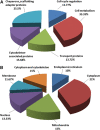
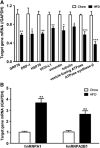
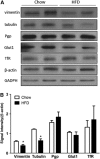
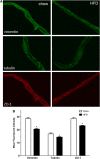
The blood-brain barrier (BBB) is a regulatory interface between the central nervous system and the rest of the body. However, BBB changes in obesity and metabolic syndrome have not been fully elucidated. We hypothesized that obesity reduces energy metabolism in the cerebral microvessels composing the BBB, reflected by downregulation of protein expression and function. We performed comparative proteomic analyses in enriched microvessels from the cerebral cortex of mice 2 months after ingestion of a high-fat diet or regular rodent chow. In mice with diet-induced obesity (DIO), there was downregulation of 47 proteins in the cerebral microvessels, including cytoskeletal proteins, chaperons, enzymes, transport-related proteins, and regulators for transcriptional and translational activities. Only two proteins, involved in messenger RNA (mRNA) transport and processing, were upregulated. The changes of these proteins were further validated by quantitative polymerase chain reaction (qPCR), western blotting, and immunofluorescent staining of freshly isolated microvessels, in samples obtained from different batches of mice. The predominant downregulation suggests that DIO suppresses metabolic activity of BBB microvessels. The finding of a hypometabolic state of the BBB in mice at the chronic stage of DIO is unexpected and unprecedented; it may provide novel mechanistic insight into how obesity influences CNS function via regulatory changes of the BBB.
Figures
Similar articles
-
Endothelial Adora2a Activation Promotes Blood-Brain Barrier Breakdown and Cognitive Impairment in Mice with Diet-Induced Insulin Resistance.J Neurosci. 2019 May 22;39(21):4179-4192. doi: 10.1523/JNEUROSCI.2506-18.2019. Epub 2019 Mar 18. J Neurosci. 2019. PMID: 30886019 Free PMC article.
-
Telmisartan prevents diet-induced obesity and preserves leptin transport across the blood-brain barrier in high-fat diet-fed mice.Pflugers Arch. 2018 Nov;470(11):1673-1689. doi: 10.1007/s00424-018-2178-0. Epub 2018 Jul 5. Pflugers Arch. 2018. PMID: 29978352
-
Endothelial-specific deficiency of megalin in the brain protects mice against high-fat diet challenge.J Neuroinflammation. 2020 Jan 14;17(1):22. doi: 10.1186/s12974-020-1702-2. J Neuroinflammation. 2020. PMID: 31937343 Free PMC article.
-
Differential regulation of pancreatic digestive enzymes during chronic high-fat diet-induced obesity in C57BL/6J mice.Br J Nutr. 2014 Jul 28;112(2):154-61. doi: 10.1017/S0007114514000816. Epub 2014 May 12. Br J Nutr. 2014. PMID: 24816161
-
Proteome of the Luminal Surface of the Blood-Brain Barrier.Proteomes. 2021 Nov 10;9(4):45. doi: 10.3390/proteomes9040045. Proteomes. 2021. PMID: 34842825 Free PMC article. Review.
Cited by
-
Systemic Oxidative Stress: A key Point in Neurodegeneration - A Review.J Nutr Health Aging. 2019;23(8):694-699. doi: 10.1007/s12603-019-1240-8. J Nutr Health Aging. 2019. PMID: 31560025 Review.
-
Leukocyte infiltration into spinal cord of EAE mice is attenuated by removal of endothelial leptin signaling.Brain Behav Immun. 2014 Aug;40:61-73. doi: 10.1016/j.bbi.2014.02.003. Epub 2014 Feb 24. Brain Behav Immun. 2014. PMID: 24576482 Free PMC article.
-
Endothelial Adora2a Activation Promotes Blood-Brain Barrier Breakdown and Cognitive Impairment in Mice with Diet-Induced Insulin Resistance.J Neurosci. 2019 May 22;39(21):4179-4192. doi: 10.1523/JNEUROSCI.2506-18.2019. Epub 2019 Mar 18. J Neurosci. 2019. PMID: 30886019 Free PMC article.
-
The Relationship Between the Blood-Brain-Barrier and the Central Effects of Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter-2 Inhibitors.Diabetes Metab Syndr Obes. 2022 Aug 22;15:2583-2597. doi: 10.2147/DMSO.S375559. eCollection 2022. Diabetes Metab Syndr Obes. 2022. PMID: 36035518 Free PMC article. Review.
-
Efficient isolation of brain capillary from a single frozen mouse brain for protein expression analysis.J Cereb Blood Flow Metab. 2021 May;41(5):1026-1038. doi: 10.1177/0271678X20941449. Epub 2020 Jul 23. J Cereb Blood Flow Metab. 2021. PMID: 32703112 Free PMC article.
References
-
- Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. - PubMed
-
- Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. - PubMed
-
- Insenser M, Montes-Nieto R, Vilarrasa N, Lecube A, Simó R, Vendrell J, et al. A nontargeted proteomic approach to the study of visceral and subcutaneous adipose tissue in human obesity. Mol Cell Endocrinol. 2012;363:10–19. - PubMed
-
- Perez-Perez R, Garcia-Santos E, Ortega-Delgado FJ, López JA, Camafeita E, Ricart W, et al. Attenuated metabolism is a hallmark of obesity as revealed by comparative proteomic analysis of human omental adipose tissue. Proteomics. 2012;75:783–795. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

