P-glycoprotein mediates drug resistance via a novel mechanism involving lysosomal sequestration
- PMID: 24062304
- PMCID: PMC3814770
- DOI: 10.1074/jbc.M113.514091
P-glycoprotein mediates drug resistance via a novel mechanism involving lysosomal sequestration
Abstract
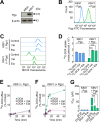
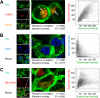
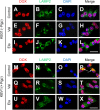
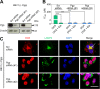
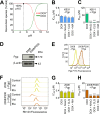
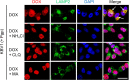
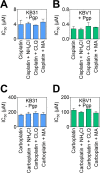
Localization of the drug transporter P-glycoprotein (Pgp) to the plasma membrane is thought to be the only contributor of Pgp-mediated multidrug resistance (MDR). However, very little work has focused on the contribution of Pgp expressed in intracellular organelles to drug resistance. This investigation describes an additional mechanism for understanding how lysosomal Pgp contributes to MDR. These studies were performed using Pgp-expressing MDR cells and their non-resistant counterparts. Using confocal microscopy and lysosomal fractionation, we demonstrated that intracellular Pgp was localized to LAMP2-stained lysosomes. In Pgp-expressing cells, the Pgp substrate doxorubicin (DOX) became sequestered in LAMP2-stained lysosomes, but this was not observed in non-Pgp-expressing cells. Moreover, lysosomal Pgp was demonstrated to be functional because DOX accumulation in this organelle was prevented upon incubation with the established Pgp inhibitors valspodar or elacridar or by silencing Pgp expression with siRNA. Importantly, to elicit drug resistance via lysosomes, the cytotoxic chemotherapeutics (e.g. DOX, daunorubicin, or vinblastine) were required to be Pgp substrates and also ionized at lysosomal pH (pH 5), resulting in them being sequestered and trapped in lysosomes. This property was demonstrated using lysosomotropic weak bases (NH4Cl, chloroquine, or methylamine) that increased lysosomal pH and sensitized only Pgp-expressing cells to such cytotoxic drugs. Consequently, a lysosomal Pgp-mediated mechanism of MDR was not found for non-ionizable Pgp substrates (e.g. colchicine or paclitaxel) or ionizable non-Pgp substrates (e.g. cisplatin or carboplatin). Together, these studies reveal a new mechanism where Pgp-mediated lysosomal sequestration of chemotherapeutics leads to MDR that is amenable to therapeutic exploitation.
Keywords: Chemical Biology; Chemoresistance; Chloroquine; Doxorubicin; Drug Resistance; Drug Transport; Lysosomes; Multidrug Resistance; P-glycoprotein.
Figures
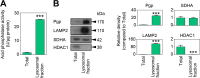

Similar articles
-
Glucose Modulation Induces Lysosome Formation and Increases Lysosomotropic Drug Sequestration via the P-Glycoprotein Drug Transporter.J Biol Chem. 2016 Feb 19;291(8):3796-820. doi: 10.1074/jbc.M115.682450. Epub 2015 Nov 24. J Biol Chem. 2016. PMID: 26601947 Free PMC article.
-
Tumor stressors induce two mechanisms of intracellular P-glycoprotein-mediated resistance that are overcome by lysosomal-targeted thiosemicarbazones.J Biol Chem. 2018 Mar 9;293(10):3562-3587. doi: 10.1074/jbc.M116.772699. Epub 2018 Jan 5. J Biol Chem. 2018. PMID: 29305422 Free PMC article.
-
Di-2-pyridylketone 4,4-dimethyl-3-thiosemicarbazone (Dp44mT) overcomes multidrug resistance by a novel mechanism involving the hijacking of lysosomal P-glycoprotein (Pgp).J Biol Chem. 2015 Apr 10;290(15):9588-603. doi: 10.1074/jbc.M114.631283. Epub 2015 Feb 26. J Biol Chem. 2015. PMID: 25720491 Free PMC article.
-
Modulation and prevention of multidrug resistance by inhibitors of P-glycoprotein.Cancer Chemother Pharmacol. 1997;40 Suppl:S13-9. doi: 10.1007/s002800051055. Cancer Chemother Pharmacol. 1997. PMID: 9272128 Review.
-
Turning the gun on cancer: Utilizing lysosomal P-glycoprotein as a new strategy to overcome multi-drug resistance.Free Radic Biol Med. 2016 Jul;96:432-45. doi: 10.1016/j.freeradbiomed.2016.04.201. Epub 2016 May 3. Free Radic Biol Med. 2016. PMID: 27154979 Review.
Cited by
-
Repurposing functional inhibitors of acid sphingomyelinase (fiasmas): an opportunity against SARS-CoV-2 infection?J Clin Pharm Ther. 2021 Oct;46(5):1213-1219. doi: 10.1111/jcpt.13390. Epub 2021 Mar 1. J Clin Pharm Ther. 2021. PMID: 33645763 Free PMC article. Review.
-
Lysosomes as a Target of Anticancer Therapy.Int J Mol Sci. 2023 Jan 22;24(3):2176. doi: 10.3390/ijms24032176. Int J Mol Sci. 2023. PMID: 36768500 Free PMC article. Review.
-
Thiosemicarbazones Can Act Synergistically with Anthracyclines to Downregulate CHEK1 Expression and Induce DNA Damage in Cell Lines Derived from Pediatric Solid Tumors.Int J Mol Sci. 2022 Aug 1;23(15):8549. doi: 10.3390/ijms23158549. Int J Mol Sci. 2022. PMID: 35955683 Free PMC article.
-
The Emerging Role of Exosomes in Cancer Chemoresistance.Front Cell Dev Biol. 2021 Oct 28;9:737962. doi: 10.3389/fcell.2021.737962. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34778252 Free PMC article. Review.
-
PEGylated Liposomes Remotely Loaded with the Combination of Doxorubicin, Quinine, and Indocyanine Green Enable Successful Treatment of Multidrug-Resistant Tumors.Pharmaceutics. 2021 Dec 17;13(12):2181. doi: 10.3390/pharmaceutics13122181. Pharmaceutics. 2021. PMID: 34959462 Free PMC article.
References
-
- Higgins C. F. (2007) Multiple molecular mechanisms for multidrug resistance transporters. Nature 446, 749–757 - PubMed
-
- Fu D., Bebawy M., Kable E. P., Roufogalis B. D. (2004) Dynamic and intracellular trafficking of P-glycoprotein-EGFP fusion protein. Implications in multidrug resistance in cancer. Int. J. Cancer 109, 174–181 - PubMed
-
- Solazzo M., Fantappiè O., Lasagna N., Sassoli C., Nosi D., Mazzanti R. (2006) P-GP localization in mitochondria and its functional characterization in multiple drug-resistant cell lines. Exp. Cell Res. 312, 4070–4078 - PubMed
-
- Baldini N., Scotlandi K., Serra M., Shikita T., Zini N., Ognibene A., Santi S., Ferracini R., Maraldi N. M. (1995) Nuclear immunolocalization of P-glycoprotein in multidrug-resistant cell lines showing similar mechanisms of doxorubicin distribution. Eur. J. Cell Biol. 68, 226–239 - PubMed
-
- Yuan J., Lovejoy D. B., Richardson D. R. (2004) Novel di-2-pyridyl-derived iron chelators with marked and selective antitumor activity. In vitro and in vivo assessment. Blood 104, 1450–1458 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

