The function of α-synuclein
- PMID: 24050397
- PMCID: PMC3866954
- DOI: 10.1016/j.neuron.2013.09.004
The function of α-synuclein
Abstract
Human genetics has indicated a causal role for the protein α-synuclein in the pathogenesis of familial Parkinson's disease (PD), and the aggregation of synuclein in essentially all patients with PD suggests a central role for this protein in the sporadic disorder. Indeed, the accumulation of misfolded α-synuclein now defines multiple forms of neural degeneration. Like many of the proteins that accumulate in other neurodegenerative disorders, however, the normal function of synuclein remains poorly understood. In this article, we review the role of synuclein at the nerve terminal and in membrane remodeling. We also consider the prion-like propagation of misfolded synuclein as a mechanism for the spread of degeneration through the neuraxis.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures

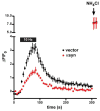
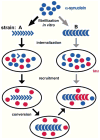

Similar articles
-
Pros and cons of a prion-like pathogenesis in Parkinson's disease.BMC Neurol. 2011 Jun 20;11:74. doi: 10.1186/1471-2377-11-74. BMC Neurol. 2011. PMID: 21689433 Free PMC article.
-
Parkinson's disease and alpha synuclein: is Parkinson's disease a prion-like disorder?Mov Disord. 2013 Jan;28(1):31-40. doi: 10.1002/mds.25373. Mov Disord. 2013. PMID: 23390095 Review.
-
Copper and iron ions accelerate the prion-like propagation of α-synuclein: A vicious cycle in Parkinson's disease.Int J Biol Macromol. 2020 Nov 15;163:562-573. doi: 10.1016/j.ijbiomac.2020.06.274. Epub 2020 Jul 3. Int J Biol Macromol. 2020. PMID: 32629061
-
[Prion Properties of Alpha-Synuclein].Mol Biol (Mosk). 2019 May-Jun;53(3):380-387. doi: 10.1134/S0026898419030182. Mol Biol (Mosk). 2019. PMID: 31184602 Review. Russian.
-
Cell Biology and Pathophysiology of α-Synuclein.Cold Spring Harb Perspect Med. 2018 Mar 1;8(3):a024091. doi: 10.1101/cshperspect.a024091. Cold Spring Harb Perspect Med. 2018. PMID: 28108534 Free PMC article. Review.
Cited by
-
Cathepsin L-containing exosomes from α-synuclein-activated microglia induce neurotoxicity through the P2X7 receptor.NPJ Parkinsons Dis. 2022 Oct 6;8(1):127. doi: 10.1038/s41531-022-00394-9. NPJ Parkinsons Dis. 2022. PMID: 36202834 Free PMC article.
-
α-Synuclein expression in the mouse cerebellum is restricted to VGluT1 excitatory terminals and is enriched in unipolar brush cells.Cerebellum. 2015 Oct;14(5):516-27. doi: 10.1007/s12311-015-0673-9. Cerebellum. 2015. PMID: 25917213 Free PMC article.
-
Multiple system atrophy: α-Synuclein strains at the neuron-oligodendrocyte crossroad.Mol Neurodegener. 2022 Nov 26;17(1):77. doi: 10.1186/s13024-022-00579-z. Mol Neurodegener. 2022. PMID: 36435784 Free PMC article. Review.
-
Toward the quantification of α-synuclein aggregates with digital seed amplification assays.Proc Natl Acad Sci U S A. 2024 Jan 16;121(3):e2312031121. doi: 10.1073/pnas.2312031121. Epub 2024 Jan 9. Proc Natl Acad Sci U S A. 2024. PMID: 38194461 Free PMC article.
-
A dual target molecular magnetic resonance imaging probe for noninvasive profiling of pathologic alpha-synuclein and microgliosis in a mouse model of Parkinson's disease.Front Neurosci. 2024 Jul 24;18:1428736. doi: 10.3389/fnins.2024.1428736. eCollection 2024. Front Neurosci. 2024. PMID: 39114484 Free PMC article.
References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, et al. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
-
- Ahlskog JE. Beating a dead horse: dopamine and Parkinson disease. Neurology. 2007;69:1701–1711. - PubMed
-
- Ahn TB, Kim SY, Kim JY, Park SS, Lee DS, Min HJ, Kim YK, Kim SE, Kim JM, Kim HJ, et al. alpha-Synuclein gene duplication is present in sporadic Parkinson disease. Neurology. 2008;70:43–49. - PubMed
-
- Alim MA, Hossain MS, Arima K, Takeda K, Izumiyama Y, Nakamura M, Kaji H, Shinoda T, Hisanaga S, Ueda K. Tubulin seeds alpha-synuclein fibril formation. J Biol Chem. 2002;277:2112–2117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

