Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration
- PMID: 24046746
- PMCID: PMC3764375
- DOI: 10.3389/fnagi.2013.00048
Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration
Abstract
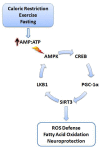
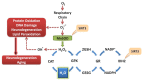
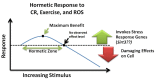
Caloric restriction (CR), fasting, and exercise have long been recognized for their neuroprotective and lifespan-extending properties; however, the underlying mechanisms of these phenomena remain elusive. Such extraordinary benefits might be linked to the activation of sirtuins. In mammals, the sirtuin family has seven members (SIRT1-7), which diverge in tissue distribution, subcellular localization, enzymatic activity, and targets. SIRT1, SIRT2, and SIRT3 have deacetylase activity. Their dependence on NAD(+) directly links their activity to the metabolic status of the cell. High NAD(+) levels convey neuroprotective effects, possibly via activation of sirtuin family members. Mitochondrial sirtuin 3 (SIRT3) has received much attention for its role in metabolism and aging. Specific small nucleotide polymorphisms in Sirt3 are linked to increased human lifespan. SIRT3 mediates the adaptation of increased energy demand during CR, fasting, and exercise to increased production of energy equivalents. SIRT3 deacetylates and activates mitochondrial enzymes involved in fatty acid β-oxidation, amino acid metabolism, the electron transport chain, and antioxidant defenses. As a result, the mitochondrial energy metabolism increases. In addition, SIRT3 prevents apoptosis by lowering reactive oxygen species and inhibiting components of the mitochondrial permeability transition pore. Mitochondrial deficits associated with aging and neurodegeneration might therefore be slowed or even prevented by SIRT3 activation. In addition, upregulating SIRT3 activity by dietary supplementation of sirtuin activating compounds might promote the beneficial effects of this enzyme. The goal of this review is to summarize emerging data supporting a neuroprotective action of SIRT3 against Alzheimer's disease, Huntington's disease, Parkinson's disease, and amyotrophic lateral sclerosis.
Keywords: SIRT3; aging; antioxidants; caloric restriction; mitochondria; neurodegeneration; neuroprotection.
Figures
Similar articles
-
Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease.Aging Cell. 2017 Feb;16(1):4-16. doi: 10.1111/acel.12538. Epub 2016 Sep 29. Aging Cell. 2017. PMID: 27686535 Free PMC article. Review.
-
SIRT3 deficiency decreases oxidative metabolism capacity but increases lifespan in male mice under caloric restriction.Aging Cell. 2022 Dec;21(12):e13721. doi: 10.1111/acel.13721. Epub 2022 Oct 5. Aging Cell. 2022. PMID: 36199173 Free PMC article.
-
Obesity and aging diminish sirtuin 1 (SIRT1)-mediated deacetylation of SIRT3, leading to hyperacetylation and decreased activity and stability of SIRT3.J Biol Chem. 2017 Oct 20;292(42):17312-17323. doi: 10.1074/jbc.M117.778720. Epub 2017 Aug 14. J Biol Chem. 2017. PMID: 28808064 Free PMC article.
-
SIRT3 regulates mitochondrial protein acetylation and intermediary metabolism.Cold Spring Harb Symp Quant Biol. 2011;76:267-77. doi: 10.1101/sqb.2011.76.010850. Epub 2011 Nov 23. Cold Spring Harb Symp Quant Biol. 2011. PMID: 22114326 Review.
-
SIRT3 and mitochondrial metabolism in neurodegenerative diseases.Neurochem Int. 2017 Oct;109:184-192. doi: 10.1016/j.neuint.2017.04.012. Epub 2017 Apr 25. Neurochem Int. 2017. PMID: 28449871 Review.
Cited by
-
Curcumin elevates sirtuin level but does not postpone in vitro senescence of human cells building the vasculature.Oncotarget. 2016 Apr 12;7(15):19201-13. doi: 10.18632/oncotarget.8450. Oncotarget. 2016. PMID: 27034011 Free PMC article.
-
Roles of Mitochondrial Sirtuins in Mitochondrial Function, Redox Homeostasis, Insulin Resistance and Type 2 Diabetes.Int J Mol Sci. 2020 Jul 24;21(15):5266. doi: 10.3390/ijms21155266. Int J Mol Sci. 2020. PMID: 32722262 Free PMC article. Review.
-
Function of the SIRT3 mitochondrial deacetylase in cellular physiology, cancer, and neurodegenerative disease.Aging Cell. 2017 Feb;16(1):4-16. doi: 10.1111/acel.12538. Epub 2016 Sep 29. Aging Cell. 2017. PMID: 27686535 Free PMC article. Review.
-
Microglia mediated neuroinflammation - signaling regulation and therapeutic considerations with special reference to some natural compounds.Histol Histopathol. 2020 Nov;35(11):1229-1250. doi: 10.14670/HH-18-239. Epub 2020 Jul 14. Histol Histopathol. 2020. PMID: 32662061 Review.
-
Potential role of exercise-induced glucose-6-phosphate isomerase in skeletal muscle function.J Exerc Nutrition Biochem. 2019 Jun 30;23(2):28-33. doi: 10.20463/jenb.2019.0014. J Exerc Nutrition Biochem. 2019. PMID: 31337203 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

