Crenolanib is active against models of drug-resistant FLT3-ITD-positive acute myeloid leukemia
- PMID: 24046014
- PMCID: PMC3837509
- DOI: 10.1182/blood-2013-07-513044
Crenolanib is active against models of drug-resistant FLT3-ITD-positive acute myeloid leukemia
Abstract
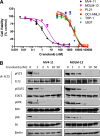
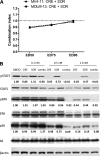
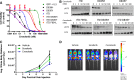
FLT3 kinase internal tandem duplication (ITD) mutations are common in acute myeloid leukemia (AML) and are associated with poor clinical outcomes. Although initial responses to FLT3 tyrosine kinase inhibitors (TKIs) are observed in FLT3-ITD-positive patients, subsequent relapse often occurs upon acquisition of secondary FLT3 kinase domain (KD) mutations, primarily at residues D835 and F691. Using biochemical assays, we determined that crenolanib, a novel TKI, demonstrates type I properties and is active against FLT3 containing ITD and/or D835- or F691-activating mutations. Potent activity was observed in FLT3-ITD-positive AML cell lines. Crenolanib delayed the outgrowth of MV4-11 cells in a xenograft mouse model, whereas in combination with the type II TKI sorafenib, a significant decrease in leukemic burden (P < .001) and prolonged survival (P < .01) was observed compared with either type I or II TKI alone. Crenolanib was active against Ba/F3 cells harboring FLT3-ITD and secondary KD mutations and sorafenib-resistant MOLM-13 cells containing FLT3-ITD/D835Y both in vitro and in vivo. In addition, crenolanib inhibited drug-resistant AML primary blasts with FLT3-ITD and D835H/Y mutations. These preclinical data demonstrate that crenolanib is effective against FLT3-ITD containing secondary KD mutations, suggesting that crenolanib may be a useful therapeutic agent for TKI-naive and drug-resistant FLT3-ITD-positive AML.
Figures



Comment in
-
Emergence of crenolanib for FLT3-mutant AML.Blood. 2013 Nov 21;122(22):3547-8. doi: 10.1182/blood-2013-10-528992. Blood. 2013. PMID: 24263951
Similar articles
-
Reversal of acquired drug resistance in FLT3-mutated acute myeloid leukemia cells via distinct drug combination strategies.Clin Cancer Res. 2014 May 1;20(9):2363-74. doi: 10.1158/1078-0432.CCR-13-2052. Epub 2014 Mar 11. Clin Cancer Res. 2014. PMID: 24619500 Free PMC article.
-
Emergence of polyclonal FLT3 tyrosine kinase domain mutations during sequential therapy with sorafenib and sunitinib in FLT3-ITD-positive acute myeloid leukemia.Clin Cancer Res. 2013 Oct 15;19(20):5758-68. doi: 10.1158/1078-0432.CCR-13-1323. Epub 2013 Aug 22. Clin Cancer Res. 2013. PMID: 23969938 Free PMC article.
-
Preclinical and Pilot Study of Type I FLT3 Tyrosine Kinase Inhibitor, Crenolanib, with Sorafenib in Acute Myeloid Leukemia and FLT3-Internal Tandem Duplication.Clin Cancer Res. 2022 Jun 13;28(12):2536-2546. doi: 10.1158/1078-0432.CCR-21-4450. Clin Cancer Res. 2022. PMID: 35344039 Free PMC article.
-
Investigational FMS-like tyrosine kinase 3 inhibitors in treatment of acute myeloid leukemia.Expert Opin Investig Drugs. 2014 Jul;23(7):943-54. doi: 10.1517/13543784.2014.911839. Epub 2014 Apr 21. Expert Opin Investig Drugs. 2014. PMID: 24749672 Free PMC article. Review.
-
FLT3 inhibitors in the treatment of acute myeloid leukemia: current status and future perspectives.Minerva Med. 2020 Oct;111(5):427-442. doi: 10.23736/S0026-4806.20.06989-X. Epub 2020 Sep 21. Minerva Med. 2020. PMID: 32955823 Review.
Cited by
-
Chemotherapy Options for Poor Responders to Neoadjuvant Chemotherapy for Orbital Granulocytic Sarcoma.Curr Treat Options Oncol. 2016 Jul;17(7):38. doi: 10.1007/s11864-016-0411-7. Curr Treat Options Oncol. 2016. PMID: 27300546 Review.
-
A Novel 2-Carbon-Linked Dimeric Artemisinin With Potent Antileukemic Activity and Favorable Pharmacology.Front Oncol. 2022 Jan 11;11:790037. doi: 10.3389/fonc.2021.790037. eCollection 2021. Front Oncol. 2022. PMID: 35127495 Free PMC article.
-
Rational Design, Synthesis and Biological Evaluation of Pyrimidine-4,6-diamine derivatives as Type-II inhibitors of FLT3 Selective Against c-KIT.Sci Rep. 2018 Feb 27;8(1):3722. doi: 10.1038/s41598-018-21839-3. Sci Rep. 2018. PMID: 29487300 Free PMC article.
-
Targeting FLT3 to treat leukemia.Expert Opin Ther Targets. 2015 Jan;19(1):37-54. doi: 10.1517/14728222.2014.960843. Epub 2014 Sep 18. Expert Opin Ther Targets. 2015. PMID: 25231999 Free PMC article. Review.
-
Indole-based FLT3 inhibitors and related scaffolds as potential therapeutic agents for acute myeloid leukemia.BMC Chem. 2023 Jul 12;17(1):73. doi: 10.1186/s13065-023-00981-8. BMC Chem. 2023. PMID: 37438819 Free PMC article. Review.
References
-
- Burnett AK, Hills RK, Milligan D, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011;29(4):369–377. - PubMed
-
- Creutzig U, van den Heuvel-Eibrink MM, Gibson B, et al. AML Committee of the International BFM Study Group. Diagnosis and management of acute myeloid leukemia in children and adolescents: recommendations from an international expert panel. Blood. 2012;120(16):3187–3205. - PubMed
-
- Ferrara F, Schiffer CA. Acute myeloid leukaemia in adults. Lancet. 2013;381(9865):484–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

