HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice
- PMID: 24043801
- PMCID: PMC3799352
- DOI: 10.1073/pnas.1315295110
HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice
Abstract
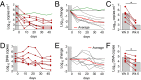
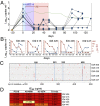
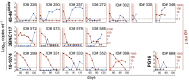
Effective control of HIV-1 infection in humans is achieved using combinations of antiretroviral therapy (ART) drugs. In humanized mice (hu-mice), control of viremia can be achieved using either ART or by immunotherapy using combinations of broadly neutralizing antibodies (bNAbs). Here we show that treatment of HIV-1-infected hu-mice with a combination of three highly potent bNAbs not only resulted in complete viremic control but also led to a reduction in cell-associated HIV-1 DNA. Moreover, lowering the initial viral load by coadministration of ART and immunotherapy enabled prolonged viremic control by a single bNAb after ART was withdrawn. Similarly, a single injection of adeno-associated virus directing expression of one bNAb produced durable viremic control after ART was terminated. We conclude that immunotherapy reduces plasma viral load and cell-associated HIV-1 DNA and that decreasing the initial viral load enables single bNAbs to control viremia in hu-mice.
Keywords: CD4bs; glycan; gp160.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
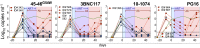
Similar articles
-
Coexistence of potent HIV-1 broadly neutralizing antibodies and antibody-sensitive viruses in a viremic controller.Sci Transl Med. 2017 Jan 18;9(373):eaal2144. doi: 10.1126/scitranslmed.aal2144. Sci Transl Med. 2017. PMID: 28100831 Free PMC article.
-
Of Mice, Macaques, and Men: Broadly Neutralizing Antibody Immunotherapy for HIV-1.Cell Host Microbe. 2017 Aug 9;22(2):207-216. doi: 10.1016/j.chom.2017.07.010. Cell Host Microbe. 2017. PMID: 28799906 Free PMC article. Review.
-
Broadly neutralizing antibody and the HIV reservoir in acute HIV infection: a strategy toward HIV remission?Curr Opin HIV AIDS. 2015 May;10(3):198-206. doi: 10.1097/COH.0000000000000144. Curr Opin HIV AIDS. 2015. PMID: 25700203 Free PMC article. Review.
-
Safety and antiviral activity of combination HIV-1 broadly neutralizing antibodies in viremic individuals.Nat Med. 2018 Nov;24(11):1701-1707. doi: 10.1038/s41591-018-0186-4. Epub 2018 Sep 26. Nat Med. 2018. PMID: 30258217 Free PMC article. Clinical Trial.
-
Tandem bispecific neutralizing antibody eliminates HIV-1 infection in humanized mice.J Clin Invest. 2018 Jun 1;128(6):2239-2251. doi: 10.1172/JCI96764. Epub 2018 Apr 23. J Clin Invest. 2018. PMID: 29461979 Free PMC article.
Cited by
-
Humanized mice for HIV and AIDS research.Curr Opin Virol. 2016 Aug;19:56-64. doi: 10.1016/j.coviro.2016.06.010. Epub 2016 Jul 19. Curr Opin Virol. 2016. PMID: 27447446 Free PMC article. Review.
-
HIV-1 Vpu restricts Fc-mediated effector functions in vivo.Cell Rep. 2022 Nov 8;41(6):111624. doi: 10.1016/j.celrep.2022.111624. Cell Rep. 2022. PMID: 36351384 Free PMC article.
-
AAV-Delivered Antibody Mediates Significant Protective Effects against SIVmac239 Challenge in the Absence of Neutralizing Activity.PLoS Pathog. 2015 Aug 6;11(8):e1005090. doi: 10.1371/journal.ppat.1005090. eCollection 2015 Aug. PLoS Pathog. 2015. PMID: 26248318 Free PMC article.
-
Inhibitory Effect of Individual or Combinations of Broadly Neutralizing Antibodies and Antiviral Reagents against Cell-Free and Cell-to-Cell HIV-1 Transmission.J Virol. 2015 Aug;89(15):7813-28. doi: 10.1128/JVI.00783-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995259 Free PMC article.
-
Antibody-mediated prevention and treatment of HIV-1 infection.Retrovirology. 2018 Nov 16;15(1):73. doi: 10.1186/s12977-018-0455-9. Retrovirology. 2018. PMID: 30445968 Free PMC article. Review.
References
-
- Molina J-M, et al. CASTLE Study Team Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J Acquir Immune Defic Syndr. 2010;53(3):323–332. - PubMed
-
- Bartlett JA, DeMasi R, Quinn J, Moxham C, Rousseau F. Overview of the effectiveness of triple combination therapy in antiretroviral-naive HIV-1 infected adults. AIDS. 2001;15(11):1369–1377. - PubMed
-
- Sigal A, et al. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature. 2011;477(7362):95–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

