Host programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-versus-host disease lethality
- PMID: 24030385
- PMCID: PMC3811178
- DOI: 10.1182/blood-2013-05-500801
Host programmed death ligand 1 is dominant over programmed death ligand 2 expression in regulating graft-versus-host disease lethality
Abstract
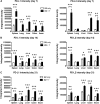
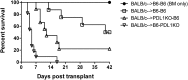
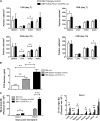
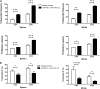
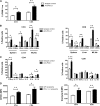
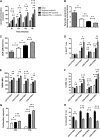
Programmed death 1 (PD-1) and its ligands, PD-L1 and PD-L2, play an important role in the maintenance of peripheral tolerance. We explored the role of PD-1 ligands in regulating graft-versus-host disease (GVHD). Both PD-L1 and PD-L2 expression were upregulated in the spleen, liver, colon, and ileum of GVHD mice. Whereas PD-L2 expression was limited to hematopoietic cells, hematopoietic and endothelial cells expressed PD-L1. PD-1/PD-L1, but not PD-1/PD-L2, blockade markedly accelerated GVHD-induced lethality. Chimera studies suggest that PD-L1 expression on host parenchymal cells is more critical than hematopoietic cells in regulating acute GVHD. Rapid mortality onset in PD-L1-deficient hosts was associated with increased gut T-cell homing and loss of intestinal epithelial integrity, along with increased donor T-cell proliferation, activation, Th1 cytokine production, and reduced apoptosis. Bioenergetics profile analysis of proliferating alloreactive donor T-cells demonstrated increased aerobic glycolysis and oxidative phosphorylation in PD-L1-deficient hosts. Donor T-cells exhibited a hyperpolarized mitochondrial membrane potential, increased superoxide production, and increased expression of a glucose transporter in PD-L1-deficient hosts. Taken together, these data provide new insight into the differential roles of host PD-L1 and PD-L2 and their associated cellular and metabolic mechanisms controlling acute GVHD.
Figures

Similar articles
-
Programmed death ligand-1 expression on donor T cells drives graft-versus-host disease lethality.J Clin Invest. 2016 Jul 1;126(7):2642-60. doi: 10.1172/JCI85796. Epub 2016 Jun 13. J Clin Invest. 2016. PMID: 27294527 Free PMC article.
-
PD-L1 Prevents the Development of Autoimmune Heart Disease in Graft-versus-Host Disease.J Immunol. 2018 Jan 15;200(2):834-846. doi: 10.4049/jimmunol.1701076. Epub 2017 Dec 6. J Immunol. 2018. PMID: 29212909 Free PMC article.
-
Programmed death-1 pathway in host tissues ameliorates Th17/Th1-mediated experimental chronic graft-versus-host disease.J Immunol. 2014 Sep 1;193(5):2565-73. doi: 10.4049/jimmunol.1400954. Epub 2014 Jul 30. J Immunol. 2014. PMID: 25080485
-
[Progress in PD-1/PD-L1, PD-L2 signaling pathway and its role in host anti-tuberculosis immunity].Zhonghua Jie He He Hu Xi Za Zhi. 2024 May 12;47(5):485-489. doi: 10.3760/cma.j.cn112147-20230904-00133. Zhonghua Jie He He Hu Xi Za Zhi. 2024. PMID: 38706074 Review. Chinese.
-
Regulation of GVHD and GVL Activity via PD-L1 Interaction With PD-1 and CD80.Front Immunol. 2018 Dec 21;9:3061. doi: 10.3389/fimmu.2018.03061. eCollection 2018. Front Immunol. 2018. PMID: 30622541 Free PMC article. Review.
Cited by
-
Clinical significance of T cell metabolic reprogramming in cancer.Clin Transl Med. 2016 Dec;5(1):29. doi: 10.1186/s40169-016-0110-9. Epub 2016 Aug 10. Clin Transl Med. 2016. PMID: 27510264 Free PMC article. Review.
-
T cell metabolism in graft-versus-host disease.Blood Sci. 2020 Jan 16;2(1):16-21. doi: 10.1097/BS9.0000000000000035. eCollection 2020 Jan. Blood Sci. 2020. PMID: 35399863 Free PMC article. Review.
-
PTCy ameliorates GVHD by restoring regulatory and effector T-cell homeostasis in recipients with PD-1 blockade.Blood Adv. 2019 Dec 10;3(23):4081-4094. doi: 10.1182/bloodadvances.2019000134. Blood Adv. 2019. PMID: 31821459 Free PMC article.
-
[Tole of T cell exhaustion in inducing immune tolerance and preventing graft-versus-host disease].Zhonghua Xue Ye Xue Za Zhi. 2019 Feb 14;40(2):164-167. doi: 10.3760/cma.j.issn.0253-2727.2019.02.015. Zhonghua Xue Ye Xue Za Zhi. 2019. PMID: 30831636 Free PMC article. Chinese. No abstract available.
-
PD-L1 siRNA-mediated silencing in acute myeloid leukemia enhances anti-leukemic T cell reactivity.Bone Marrow Transplant. 2020 Dec;55(12):2308-2318. doi: 10.1038/s41409-020-0966-6. Epub 2020 Jun 11. Bone Marrow Transplant. 2020. PMID: 32528120
References
-
- Sheppard KA, Fitz LJ, Lee JM, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574(1-3):37–41. - PubMed
-
- Latchman Y, Wood CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AI056299/AI/NIAID NIH HHS/United States
- S10 RR016851/RR/NCRR NIH HHS/United States
- P01 CA065493/CA/NCI NIH HHS/United States
- R37 AI034495/AI/NIAID NIH HHS/United States
- HL056067/HL/NHLBI NIH HHS/United States
- P01 CA142106/CA/NCI NIH HHS/United States
- CA072669/CA/NCI NIH HHS/United States
- R01 HL049997/HL/NHLBI NIH HHS/United States
- R01 AI034495/AI/NIAID NIH HHS/United States
- HL049997/HL/NHLBI NIH HHS/United States
- R37 HL056067/HL/NHLBI NIH HHS/United States
- R01 HL056067/HL/NHLBI NIH HHS/United States
- AI056299/AI/NIAID NIH HHS/United States
- AI034495/AI/NIAID NIH HHS/United States
- R01 CA072669/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

