The impact of α-toxin on host cell plasma membrane permeability and cytokine expression during human blood infection by CA-MRSA USA300
- PMID: 24026286
- PMCID: PMC3800068
- DOI: 10.1189/jlb.0213080
The impact of α-toxin on host cell plasma membrane permeability and cytokine expression during human blood infection by CA-MRSA USA300
Abstract
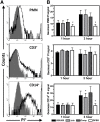
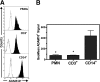
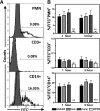
This investigation examines the influence of α-toxin (Hla) expression by CA-MRSA on host immune cell integrity and cytokine expression during infection of human blood. Flow cytometry analysis of human blood infected by Staphylococcus aureus PFGE type USA300 or a USA300Δhla demonstrated that Hla expression significantly increased plasma membrane permeability of human CD14(+) monocytes. The increased susceptibility of human CD14(+) monocytes to Hla toxicity paralleled the high cell-surface expression on these cell types of ADAM10. USA300 rapidly associated with PMNs and monocytes but not T cells following inoculation of human blood. Transcription analysis indicated a strong up-regulation of proinflammatory cytokine transcription following infection of human blood by USA300 and USA300Δhla. CBAs and ELISAs determined that IL-6, IL-10, TNF-α, IFN-γ, IL-1β, IL-8, and IL-4 are significantly up-regulated during the initial phases of human blood infection by USA300 relative to mock-infected blood but failed to distinguish any significant differences in secreted cytokine protein concentrations during infection by USA300Δhla relative to USA300. Collectively, these findings demonstrate that expression of Hla by USA300 has a significant impact on human CD14(+) monocyte plasma membrane integrity but is not exclusively responsible for the proinflammatory cytokine profile induced by USA300 during the initial stages of human blood infection.
Keywords: Staphylococcus aureus; T cell; chemokine; monocyte; pathogenesis; virulence.
Figures
Similar articles
-
Alpha-toxin induces programmed cell death of human T cells, B cells, and monocytes during USA300 infection.PLoS One. 2012;7(5):e36532. doi: 10.1371/journal.pone.0036532. Epub 2012 May 4. PLoS One. 2012. PMID: 22574180 Free PMC article.
-
The adherens junctions control susceptibility to Staphylococcus aureus α-toxin.Proc Natl Acad Sci U S A. 2015 Nov 17;112(46):14337-42. doi: 10.1073/pnas.1510265112. Epub 2015 Oct 21. Proc Natl Acad Sci U S A. 2015. PMID: 26489655 Free PMC article.
-
ADAM10 Cell Surface Expression but Not Activity Is Critical for Staphylococcus aureus α-Hemolysin-Mediated Activation of the NLRP3 Inflammasome in Human Monocytes.Toxins (Basel). 2016 Mar 30;8(4):95. doi: 10.3390/toxins8040095. Toxins (Basel). 2016. PMID: 27043625 Free PMC article.
-
Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus.Annu Rev Microbiol. 2010;64:143-62. doi: 10.1146/annurev.micro.112408.134309. Annu Rev Microbiol. 2010. PMID: 20825344 Review.
-
Virulence strategies of the dominant USA300 lineage of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA).FEMS Immunol Med Microbiol. 2012 Jun;65(1):5-22. doi: 10.1111/j.1574-695X.2012.00937.x. Epub 2012 Mar 5. FEMS Immunol Med Microbiol. 2012. PMID: 22309135 Free PMC article. Review.
Cited by
-
Exotoxins from Staphylococcus aureus activate 5-lipoxygenase and induce leukotriene biosynthesis.Cell Mol Life Sci. 2020 Oct;77(19):3841-3858. doi: 10.1007/s00018-019-03393-x. Epub 2019 Dec 5. Cell Mol Life Sci. 2020. PMID: 31807813 Free PMC article.
-
Untargeted lipidomic analysis to broadly characterize the effects of pathogenic and non-pathogenic staphylococci on mammalian lipids.PLoS One. 2018 Oct 31;13(10):e0206606. doi: 10.1371/journal.pone.0206606. eCollection 2018. PLoS One. 2018. PMID: 30379915 Free PMC article.
-
Interaction of Staphylococci with Human B cells.PLoS One. 2016 Oct 6;11(10):e0164410. doi: 10.1371/journal.pone.0164410. eCollection 2016. PLoS One. 2016. PMID: 27711145 Free PMC article.
-
Aspartic Acid Residue 51 of SaeR Is Essential for Staphylococcus aureus Virulence.Front Microbiol. 2018 Dec 14;9:3085. doi: 10.3389/fmicb.2018.03085. eCollection 2018. Front Microbiol. 2018. PMID: 30619166 Free PMC article.
-
Epic Immune Battles of History: Neutrophils vs. Staphylococcus aureus.Front Cell Infect Microbiol. 2017 Jun 30;7:286. doi: 10.3389/fcimb.2017.00286. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28713774 Free PMC article. Review.
References
-
- Klevens R. M., Morrison M. A., Nadle J., Petit S., Gershman K., Ray S., Harrison L. H., Lynfield R., Dumyati G., Townes J. M., Craig A. S., Zell E. R., Fosheim G. E., McDougal L. K., Carey R. B., Fridkin S. K., Active Bacterial Core Surveillance (ABCs) MRSA Investigators (2007) Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298, 1763–1771 - PubMed
-
- Nygaard T. K., DeLeo F. R., Voyich J. M. (2008) Community-associated methicillin-resistant Staphylococcus aureus skin infections: advances toward identifying the key virulence factors. Curr. Opin. Infect. Dis. 21, 147–152 - PubMed
-
- Moran G. J., Krishnadasan A., Gorwitz R. J., Fosheim G. E., McDougal L. K., Carey R. B., Talan D. A., EMERGEncy ID Net Study Group (2006) Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355, 666–674 - PubMed
-
- Voyich J. M., Braughton K. R., Sturdevant D. E., Whitney A. R., Said-Salim B., Porcella S. F., Long R. D., Dorward D. W., Gardner D. J., Kreiswirth B. N., Musser J. M., DeLeo F. R. (2005) Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J. Immunol. 175, 3907–3919 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

