A type III effector antagonizes death receptor signalling during bacterial gut infection
- PMID: 24025841
- PMCID: PMC3836246
- DOI: 10.1038/nature12524
A type III effector antagonizes death receptor signalling during bacterial gut infection
Abstract
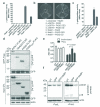
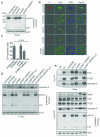
Successful infection by enteric bacterial pathogens depends on the ability of the bacteria to colonize the gut, replicate in host tissues and disseminate to other hosts. Pathogens such as Salmonella, Shigella and enteropathogenic and enterohaemorrhagic (EPEC and EHEC, respectively) Escherichia coli use a type III secretion system (T3SS) to deliver virulence effector proteins into host cells during infection that promote colonization and interfere with antimicrobial host responses. Here we report that the T3SS effector NleB1 from EPEC binds to host cell death-domain-containing proteins and thereby inhibits death receptor signalling. Protein interaction studies identified FADD, TRADD and RIPK1 as binding partners of NleB1. NleB1 expressed ectopically or injected by the bacterial T3SS prevented Fas ligand or TNF-induced formation of the canonical death-inducing signalling complex (DISC) and proteolytic activation of caspase-8, an essential step in death-receptor-induced apoptosis. This inhibition depended on the N-acetylglucosamine transferase activity of NleB1, which specifically modified Arg 117 in the death domain of FADD. The importance of the death receptor apoptotic pathway to host defence was demonstrated using mice deficient in the FAS signalling pathway, which showed delayed clearance of the EPEC-like mouse pathogen Citrobacter rodentium and reversion to virulence of an nleB mutant. The activity of NleB suggests that EPEC and other attaching and effacing pathogens antagonize death-receptor-induced apoptosis of infected cells, thereby blocking a major antimicrobial host response.
Figures


Similar articles
-
Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains.Nature. 2013 Sep 12;501(7466):242-6. doi: 10.1038/nature12436. Epub 2013 Aug 18. Nature. 2013. PMID: 23955153
-
The bacterial arginine glycosyltransferase effector NleB preferentially modifies Fas-associated death domain protein (FADD).J Biol Chem. 2017 Oct 20;292(42):17337-17350. doi: 10.1074/jbc.M117.805036. Epub 2017 Aug 31. J Biol Chem. 2017. PMID: 28860194 Free PMC article.
-
Distinct Roles of the Antiapoptotic Effectors NleB and NleF from Enteropathogenic Escherichia coli.Infect Immun. 2017 Mar 23;85(4):e01071-16. doi: 10.1128/IAI.01071-16. Print 2017 Apr. Infect Immun. 2017. PMID: 28138023 Free PMC article.
-
Type Three Secretion System in Attaching and Effacing Pathogens.Front Cell Infect Microbiol. 2016 Oct 21;6:129. doi: 10.3389/fcimb.2016.00129. eCollection 2016. Front Cell Infect Microbiol. 2016. PMID: 27818950 Free PMC article. Review.
-
Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements.Mol Microbiol. 2011 Jun;80(6):1420-38. doi: 10.1111/j.1365-2958.2011.07661.x. Epub 2011 May 5. Mol Microbiol. 2011. PMID: 21488979 Review.
Cited by
-
Identification of a Distinct Substrate-binding Domain in the Bacterial Cysteine Methyltransferase Effectors NleE and OspZ.J Biol Chem. 2016 Sep 16;291(38):20149-62. doi: 10.1074/jbc.M116.734079. Epub 2016 Jul 21. J Biol Chem. 2016. PMID: 27445336 Free PMC article.
-
Calmodulin Binding Activates Chromobacterium CopC Effector to ADP-Riboxanate Host Apoptotic Caspases.mBio. 2022 Jun 28;13(3):e0069022. doi: 10.1128/mbio.00690-22. Epub 2022 Apr 21. mBio. 2022. PMID: 35446120 Free PMC article.
-
Silver-promoted solid-phase guanidinylation enables the first synthesis of arginine glycosylated Samoamide A cyclopeptide analogue.Front Chem. 2023 Jan 4;10:1040216. doi: 10.3389/fchem.2022.1040216. eCollection 2022. Front Chem. 2023. PMID: 36688048 Free PMC article.
-
CEACAM1 regulates CD8+ T cell immunity and protects from severe pathology during Citrobacter rodentium induced colitis.Gut Microbes. 2020 Nov 1;11(6):1790-1805. doi: 10.1080/19490976.2020.1775464. Epub 2020 Jun 10. Gut Microbes. 2020. PMID: 32521208 Free PMC article.
-
Porous insights: IpaH7.8 reveals crystal-clear differences of gasdermins in mice and men.Signal Transduct Target Ther. 2023 Jun 28;8(1):258. doi: 10.1038/s41392-023-01535-z. Signal Transduct Target Ther. 2023. PMID: 37380636 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

