Haematopoietic stem cell niches: new insights inspire new questions
- PMID: 24022369
- PMCID: PMC3791375
- DOI: 10.1038/emboj.2013.201
Haematopoietic stem cell niches: new insights inspire new questions
Abstract
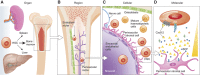
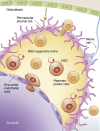
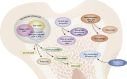
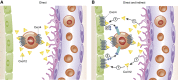
Haematopoietic stem cell (HSC) niches provide an environment essential for life-long HSC function. Intense investigation of HSC niches both feed off and drive technology development to increase our capability to assay functionally defined cells with high resolution. A major driving force behind the desire to understand the basic biology of HSC niches is the clear implications for clinical therapies. Here, with particular emphasis on cell type-specific deletion of SCL and CXCL12, we focus on unresolved issues on HSC niches, framed around some very recent advances and novel discoveries on the extrinsic regulation of HSC maintenance. We also provide ideas for possible paths forward, some of which are clearly within reach while others will require both novel tools and vision.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Differential cytokine contributions of perivascular haematopoietic stem cell niches.Nat Cell Biol. 2017 Mar;19(3):214-223. doi: 10.1038/ncb3475. Epub 2017 Feb 20. Nat Cell Biol. 2017. PMID: 28218906 Free PMC article.
-
Roles of osteoclasts in the control of medullary hematopoietic niches.Arch Biochem Biophys. 2014 Nov 1;561:29-37. doi: 10.1016/j.abb.2014.06.032. Epub 2014 Jul 3. Arch Biochem Biophys. 2014. PMID: 24998177 Review.
-
Adult haematopoietic stem cell niches.Nat Rev Immunol. 2017 Sep;17(9):573-590. doi: 10.1038/nri.2017.53. Epub 2017 Jun 12. Nat Rev Immunol. 2017. PMID: 28604734 Review.
-
CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance.Nature. 2013 Mar 14;495(7440):227-30. doi: 10.1038/nature11926. Epub 2013 Feb 24. Nature. 2013. PMID: 23434756 Free PMC article.
-
Stem cell interactions in a bone marrow niche.Curr Osteoporos Rep. 2011 Dec;9(4):210-8. doi: 10.1007/s11914-011-0075-y. Curr Osteoporos Rep. 2011. PMID: 21932020 Review.
Cited by
-
Hemmule: A Novel Structure with the Properties of the Stem Cell Niche.Int J Mol Sci. 2020 Jan 14;21(2):539. doi: 10.3390/ijms21020539. Int J Mol Sci. 2020. PMID: 31947705 Free PMC article.
-
Closer to Nature: The Role of MSCs in Recreating the Microenvironment of the Hematopoietic Stem Cell Niche in vitro.Transfus Med Hemother. 2022 Jan 14;49(4):258-267. doi: 10.1159/000520932. eCollection 2022 Aug. Transfus Med Hemother. 2022. PMID: 36159960 Free PMC article. Review.
-
A Microcavity Array-Based 4D Cell Culture Platform.Bioengineering (Basel). 2019 May 31;6(2):50. doi: 10.3390/bioengineering6020050. Bioengineering (Basel). 2019. PMID: 31159244 Free PMC article.
-
The bone metastasis niche in breast cancer-potential overlap with the haematopoietic stem cell niche in vivo.J Bone Oncol. 2019 Jun 7;17:100244. doi: 10.1016/j.jbo.2019.100244. eCollection 2019 Aug. J Bone Oncol. 2019. PMID: 31236323 Free PMC article.
-
USP10 Is an Essential Deubiquitinase for Hematopoiesis and Inhibits Apoptosis of Long-Term Hematopoietic Stem Cells.Stem Cell Reports. 2016 Dec 13;7(6):1116-1129. doi: 10.1016/j.stemcr.2016.11.003. Stem Cell Reports. 2016. PMID: 27974222 Free PMC article.
References
-
- Abkowitz JL, Robinson AE, Kale S, Long MW, Chen J (2003) Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood 102: 1249–1253 - PubMed
-
- Anderson DM, Williams DE, Tushinski R, Gimpel S, Eisenman J, Cannizzaro LA, Aronson M, Croce CM, Huebner K, Cosman D (1991) Alternate splicing of mRNAs encoding human mast cell growth factor and localization of the gene to chromosome 12q22-q24. Cell Growth Differ 2: 373–378 - PubMed
-
- Broudy VC (1997) Stem cell factor and hematopoiesis. Blood 90: 1345–1364 - PubMed
-
- Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF (2005) Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med 201: 1307–1318 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

