Gender, sex hormones and pulmonary hypertension
- PMID: 24015330
- PMCID: PMC3757824
- DOI: 10.4103/2045-8932.114756
Gender, sex hormones and pulmonary hypertension
Abstract
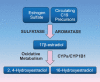
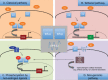
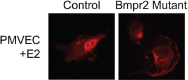
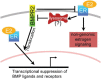
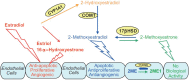
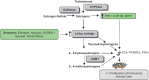
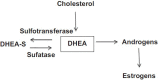
Most subtypes of pulmonary arterial hypertension (PAH) are characterized by a greater susceptibility to disease among females, although females with PAH appear to live longer after diagnosis. While this "estrogen paradoxȍ of enhanced female survival despite increased female susceptibility remains a mystery, recent progress has begun to shed light upon the interplay of sex hormones, the pathogenesis of pulmonary hypertension, and the right ventricular response to stress. For example, emerging data in humans and experimental models suggest that estrogens or differential sex hormone metabolism may modify disease risk among susceptible subjects, and that estrogens may interact with additional local factors such as serotonin to enhance the potentially damaging chronic effects of estrogens on the pulmonary vasculature. Regardless, it remains unclear why not all estrogenic compounds behave equally, nor why estrogens appear to be protective in certain settings but detrimental in others. The contribution of androgens and other compounds, such as dehydroepiandrosterone, to pathogenesis and possibly treatment must be considered as well. In this review, we will discuss the recent understandings on how estrogens, estrogen metabolism, dehydroepiandrosterone, and additional susceptibility factors may all contribute to the pathogenesis or potentially to the treatment of pulmonary hypertension, by evaluating current human, cell-based, and experimental model data.
Keywords: bone morphogenetic protein receptor type II; dehydroepiandrosterone; estrogen; pulmonary hypertension; serotonin.
Conflict of interest statement
Figures
Similar articles
-
Progress in solving the sex hormone paradox in pulmonary hypertension.Am J Physiol Lung Cell Mol Physiol. 2014 Jul 1;307(1):L7-26. doi: 10.1152/ajplung.00337.2013. Epub 2014 May 9. Am J Physiol Lung Cell Mol Physiol. 2014. PMID: 24816487 Review.
-
[Female gender and pulmonary arterial hypertension: a complex relationship].G Ital Cardiol (Rome). 2012 Jun;13(6):448-60. doi: 10.1714/1073.11764. G Ital Cardiol (Rome). 2012. PMID: 22622125 Italian.
-
Development of pulmonary arterial hypertension in women: interplay of sex hormones and pulmonary vascular disease.Womens Health (Lond). 2010 Mar;6(2):285-96. doi: 10.2217/whe.09.88. Womens Health (Lond). 2010. PMID: 20187732 Free PMC article. Review.
-
The Role of Sex in the Pathophysiology of Pulmonary Hypertension.Adv Exp Med Biol. 2018;1065:511-528. doi: 10.1007/978-3-319-77932-4_31. Adv Exp Med Biol. 2018. PMID: 30051404 Review.
-
Pulmonary arterial hypertension: Sex matters.Br J Pharmacol. 2024 Apr;181(7):938-966. doi: 10.1111/bph.16277. Epub 2024 Jan 16. Br J Pharmacol. 2024. PMID: 37939796 Review.
Cited by
-
A Systematic Review of Novel Therapies of Pulmonary Arterial Hypertension.Am J Cardiovasc Drugs. 2024 Jan;24(1):39-54. doi: 10.1007/s40256-023-00613-5. Epub 2023 Nov 9. Am J Cardiovasc Drugs. 2024. PMID: 37945977 Free PMC article.
-
Pulmonary hypertension associated mortality in the United States from 2003 to 2020: an observational analysis of time trends and disparities.J Thorac Dis. 2023 Jun 30;15(6):3256-3272. doi: 10.21037/jtd-22-1468. Epub 2023 Jun 13. J Thorac Dis. 2023. PMID: 37426148 Free PMC article.
-
Therapeutic targeting of mineralocorticoid receptors in pulmonary hypertension: Insights from basic research.Front Cardiovasc Med. 2023 Jan 30;10:1118516. doi: 10.3389/fcvm.2023.1118516. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 36793473 Free PMC article. Review.
-
Prediction of patient outcomes through social determinants of health: The Pulmonary Hypertension Association Registry (PHAR) evaluation.Pulm Circ. 2022 Jul 1;12(3):e12120. doi: 10.1002/pul2.12120. eCollection 2022 Jul. Pulm Circ. 2022. PMID: 35911181 Free PMC article.
-
Sex Differences in Pulmonary Hypertension.Front Aging. 2021 Oct 4;2:727558. doi: 10.3389/fragi.2021.727558. eCollection 2021. Front Aging. 2021. PMID: 35822006 Free PMC article. Review.
References
-
- Rich S, Rabinovitch M. Diagnosis and treatment of secondary (non-category 1) pulmonary hypertension. Circulation. 2008;118:2190–9. - PubMed
-
- Tuder RM, Abman SH, Braun T, Capron F, Stevens T, Thistlethwaite PA, et al. Development and pathology of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S3–9. - PubMed
-
- Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–54. - PubMed
-
- Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, et al. Pulmonary arterial hypertension: Baseline characteristics from the reveal registry. Chest. 2010;137:376–87. - PubMed
-
- Chin KM, Rubin LJ. Pulmonary arterial hypertension. J Am Coll Cardiol. 2008;51:1527–38. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

