Mammalian target of rapamycin and Rictor control neutrophil chemotaxis by regulating Rac/Cdc42 activity and the actin cytoskeleton
- PMID: 24006489
- PMCID: PMC3814157
- DOI: 10.1091/mbc.E13-07-0405
Mammalian target of rapamycin and Rictor control neutrophil chemotaxis by regulating Rac/Cdc42 activity and the actin cytoskeleton
Abstract
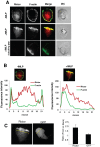
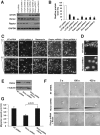
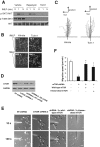
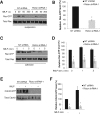
Chemotaxis allows neutrophils to seek out sites of infection and inflammation. The asymmetric accumulation of filamentous actin (F-actin) at the leading edge provides the driving force for protrusion and is essential for the development and maintenance of neutrophil polarity. The mechanism that governs actin cytoskeleton dynamics and assembly in neutrophils has been extensively explored and is still not fully understood. By using neutrophil-like HL-60 cells, we describe a pivotal role for Rictor, a component of mammalian target of rapamycin complex 2 (mTORC2), in regulating assembly of the actin cytoskeleton during neutrophil chemotaxis. Depletion of mTOR and Rictor, but not Raptor, impairs actin polymerization, leading-edge establishment, and directional migration in neutrophils stimulated with chemoattractants. Of interest, depletion of mSin1, an integral component of mTORC2, causes no detectable defects in neutrophil polarity and chemotaxis. In addition, experiments with chemical inhibition and kinase-dead mutants indicate that mTOR kinase activity and AKT phosphorylation are dispensable for chemotaxis. Instead, our results suggest that the small Rho GTPases Rac and Cdc42 serve as downstream effectors of Rictor to regulate actin assembly and organization in neutrophils. Together our findings reveal an mTORC2- and mTOR kinase-independent function and mechanism of Rictor in the regulation of neutrophil chemotaxis.
Figures

Similar articles
-
Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis.J Cell Biol. 2003 Feb 3;160(3):375-85. doi: 10.1083/jcb.200208179. Epub 2003 Jan 27. J Cell Biol. 2003. PMID: 12551955 Free PMC article.
-
Rictor regulates cell migration by suppressing RhoGDI2.Oncogene. 2013 May 16;32(20):2521-6. doi: 10.1038/onc.2012.287. Epub 2012 Jul 9. Oncogene. 2013. PMID: 22777355 Free PMC article.
-
IKK interacts with rictor and regulates mTORC2.Cell Signal. 2013 Nov;25(11):2239-45. doi: 10.1016/j.cellsig.2013.07.008. Epub 2013 Jul 17. Cell Signal. 2013. PMID: 23872070
-
Targeted Inhibition of Rictor/mTORC2 in Cancer Treatment: A New Era after Rapamycin.Curr Cancer Drug Targets. 2016;16(4):288-304. doi: 10.2174/1568009616666151113120830. Curr Cancer Drug Targets. 2016. PMID: 26563881 Review.
-
Regulation of mTOR Signaling: Emerging Role of Cyclic Nucleotide-Dependent Protein Kinases and Implications for Cardiometabolic Disease.Int J Mol Sci. 2023 Jul 15;24(14):11497. doi: 10.3390/ijms241411497. Int J Mol Sci. 2023. PMID: 37511253 Free PMC article. Review.
Cited by
-
Loss of Rictor with aging in osteoblasts promotes age-related bone loss.Cell Death Dis. 2016 Oct 13;7(10):e2408. doi: 10.1038/cddis.2016.249. Cell Death Dis. 2016. PMID: 27735936 Free PMC article.
-
Vascular hyperpermeability as a hallmark of phacomatoses: is the etiology angiogenesis related to or comparable with mechanisms seen in inflammatory pathways? Part II: angiogenesis- and inflammation-related molecular pathways, tumor-associated macrophages, and possible therapeutic implications: a comprehensive review.Neurosurg Rev. 2018 Oct;41(4):931-944. doi: 10.1007/s10143-017-0837-9. Epub 2017 Mar 11. Neurosurg Rev. 2018. PMID: 28283837 Review.
-
Mechanosensitive mTORC2 independently coordinates leading and trailing edge polarity programs during neutrophil migration.Mol Biol Cell. 2023 May 1;34(5):ar35. doi: 10.1091/mbc.E22-05-0191. Epub 2023 Mar 1. Mol Biol Cell. 2023. PMID: 36857159 Free PMC article.
-
Sex-Based Differences in Human Neutrophil Chemorepulsion.J Immunol. 2022 Jul 15;209(2):354-367. doi: 10.4049/jimmunol.2101103. Epub 2022 Jul 6. J Immunol. 2022. PMID: 35793910 Free PMC article.
-
Moving towards a paradigm: common mechanisms of chemotactic signaling in Dictyostelium and mammalian leukocytes.Cell Mol Life Sci. 2014 Oct;71(19):3711-47. doi: 10.1007/s00018-014-1638-8. Epub 2014 May 21. Cell Mol Life Sci. 2014. PMID: 24846395 Free PMC article. Review.
References
-
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. - PubMed
-
- Andrew N, Insall RH. Chemotaxis in shallow gradients is mediated independently of PtdIns 3-kinase by biased choices between random protrusions. Nat Cell Biol. 2007;9:193–200. - PubMed
-
- Bayle JH, Grimley JS, Stankunas K, Gestwicki JE, Wandless TJ, Crabtree GR. Rapamycin analogs with differential binding specificity permit orthogonal control of protein activity. Chem Biol. 2006;13:99–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

