Gemtuzumab ozogamicin can reduce minimal residual disease in patients with childhood acute myeloid leukemia
- PMID: 24006085
- PMCID: PMC4271731
- DOI: 10.1002/cncr.28334
Gemtuzumab ozogamicin can reduce minimal residual disease in patients with childhood acute myeloid leukemia
Abstract
Background: Gemtuzumab ozogamicin (GO) is an active agent for the treatment of CD33-postive acute myeloid leukemia (AML) and may improve the outcome of specific patient subgroups when combined with conventional chemotherapy. However, to the best of the authors' knowledge, the effects of GO on levels of minimal residual disease (MRD) are unknown.
Methods: Pediatric patients with AML who received GO, either alone or in combination with chemotherapy on the AML02 multicenter trial, were analyzed to determine the effects of GO on MRD and outcome.
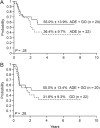
Results: Among 17 patients who received GO alone because of persistent leukemia, 14 had a reduction in their MRD level and 13 became MRD negative. Of the 29 who received chemotherapy in combination with GO after responding poorly to chemotherapy, 28 demonstrated reduced MRD and 13 became MRD negative. Treatment with GO effectively reduced MRD before hematopoietic stem cell transplantation and was not found to be associated with increased treatment-related mortality after transplantation.
Conclusions: GO is effective in reducing MRD levels in pediatric patients with AML and may improve the outcome of those patients at high risk of disease recurrence.
Keywords: acute myeloid leukemia; gemtuzumab ozogamicin; minimal residual disease; treatment-related mortality.
Copyright © 2013 American Cancer Society.
Conflict of interest statement
Drs. Inaba, Pui, and Rubnitz were supported by National Institutes of Health (NIH) grant CA21765. Dr. Pui is an American Cancer Society Professor. Dr. Campana was supported by an NIH grant and a grant from the National Medical Research Council of Singapore. He has received honoraria for solicited review articles in the
Figures


Similar articles
-
MRD assessed by WT1 and NPM1 transcript levels identifies distinct outcomes in AML patients and is influenced by gemtuzumab ozogamicin.Oncotarget. 2014 Aug 15;5(15):6280-8. doi: 10.18632/oncotarget.2196. Oncotarget. 2014. PMID: 25026287 Free PMC article. Clinical Trial.
-
Minimal residual disease-directed therapy for childhood acute myeloid leukaemia: results of the AML02 multicentre trial.Lancet Oncol. 2010 Jun;11(6):543-52. doi: 10.1016/S1470-2045(10)70090-5. Epub 2010 May 5. Lancet Oncol. 2010. PMID: 20451454 Free PMC article. Clinical Trial.
-
Fractionated doses of gemtuzumab ozogamicin combined with 3 + 7 induction chemotherapy as salvage treatment for young patients with acute myeloid leukemia in first relapse.Ann Hematol. 2012 Dec;91(12):1871-7. doi: 10.1007/s00277-012-1528-9. Epub 2012 Jul 21. Ann Hematol. 2012. PMID: 22820971
-
Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials.Lancet Oncol. 2014 Aug;15(9):986-96. doi: 10.1016/S1470-2045(14)70281-5. Epub 2014 Jul 6. Lancet Oncol. 2014. PMID: 25008258 Free PMC article. Review.
-
Efficacy and resistance of gemtuzumab ozogamicin for acute myeloid leukemia.Int J Hematol. 2013 Jun;97(6):703-16. doi: 10.1007/s12185-013-1365-1. Epub 2013 May 26. Int J Hematol. 2013. PMID: 23709007 Review.
Cited by
-
MRD assessed by WT1 and NPM1 transcript levels identifies distinct outcomes in AML patients and is influenced by gemtuzumab ozogamicin.Oncotarget. 2014 Aug 15;5(15):6280-8. doi: 10.18632/oncotarget.2196. Oncotarget. 2014. PMID: 25026287 Free PMC article. Clinical Trial.
-
Gemtuzumab ozogamicin in acute myeloid leukemia.Leukemia. 2017 Sep;31(9):1855-1868. doi: 10.1038/leu.2017.187. Epub 2017 Jun 13. Leukemia. 2017. PMID: 28607471 Review.
-
Quality of Response in Acute Myeloid Leukemia: The Role of Minimal Residual Disease.Cancers (Basel). 2019 Sep 23;11(10):1417. doi: 10.3390/cancers11101417. Cancers (Basel). 2019. PMID: 31548502 Free PMC article. Review.
-
CD33 Expression and Its Association With Gemtuzumab Ozogamicin Response: Results From the Randomized Phase III Children's Oncology Group Trial AAML0531.J Clin Oncol. 2016 Mar 1;34(7):747-55. doi: 10.1200/JCO.2015.62.6846. Epub 2016 Jan 19. J Clin Oncol. 2016. PMID: 26786921 Free PMC article. Clinical Trial.
-
Properties of Leukemic Stem Cells in Regulating Drug Resistance in Acute and Chronic Myeloid Leukemias.Biomedicines. 2022 Jul 30;10(8):1841. doi: 10.3390/biomedicines10081841. Biomedicines. 2022. PMID: 36009388 Free PMC article. Review.
References
-
- Tsukimoto I, Tawa A, Horibe K, et al. Risk-stratified therapy and the intensive use of cytarabine improves the outcome in childhood acute myeloid leukemia: the AML99 trial from the Japanese Childhood AML Cooperative Study Group. J Clin Oncol. 2009;27:4007–4013. - PubMed
-
- Gibson BE, Webb DK, Howman AJ, et al. Results of a randomized trial in children with acute myeloid leukaemia: medical research council AML12 trial. Br J Haematol. 2011;155:366–376. - PubMed
-
- Abrahamsson J, Forestier E, Heldrup J, et al. Response-guided induction therapy in pediatric acute myeloid leukemia with excellent remission rate. J Clin Oncol. 2011;29:310–315. - PubMed
-
- Cooper TM, Franklin J, Gerbing RB, et al. AAML03P1, a pilot study of the safety of gemtuzumab ozogamicin in combination with chemotherapy for newly diagnosed childhood acute myeloid leukemia: a report from the Children’s Oncology Group. Cancer. 2012;118:761–769. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

