ICAM-1-dependent homotypic aggregates regulate CD8 T cell effector function and differentiation during T cell activation
- PMID: 23997225
- PMCID: PMC3803108
- DOI: 10.4049/jimmunol.1201954
ICAM-1-dependent homotypic aggregates regulate CD8 T cell effector function and differentiation during T cell activation
Abstract
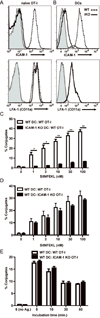
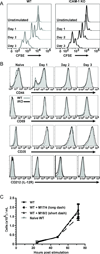
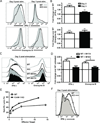
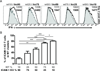
A hallmark of T cell activation in vitro and in vivo is the clustering of T cells with each other via interaction of the LFA-1 integrin with ICAM-1. The functional significance of these homotypic aggregates in regulating T cell function remains unknown. We used an APC-free in vitro activation system to demonstrate that stimulation of purified naive CD8 T cells results in enhanced expression of ICAM-1 on T cells that is sustained by the inflammatory cytokine IL-12 and associated with robust T cell aggregates. ICAM-1-deficient CD8 T cells proliferate normally but demonstrate a striking failure to aggregate. Interestingly, loss of ICAM-1 expression results in elevated levels of IFN-γ and granzyme B, as well as enhanced cytotoxicity. Similar results were obtained when anti-LFA-1 Ab was used to block the clustering of wild-type T cells. ICAM-1 ligation is not required for IFN-γ regulation, as clustering of ICAM-1-deficient CD8 T cells with wild-type T cells reduces IFN-γ expression. Analysis using a fluorescent reporter that monitors TCR signal strength indicates that T cell clustering limits T cell exposure to Ag during activation. Furthermore, T cell clustering promotes the upregulation of the CTLA-4 inhibitory receptor and the downregulation of eomesodermin, which controls effector molecule expression. Activation of ICAM-1-deficient CD8 T cells in vivo results in an enhanced percentage of KLRG-1(+) T cells indicative of short-lived effectors. These results suggest that T cell clustering represents a mechanism that allows continued proliferation but regulates T cell effector function and differentiation.
Figures





Similar articles
-
Regulatory T-cell development and function are impaired in mice lacking membrane expression of full length intercellular adhesion molecule-1.Immunology. 2015 Dec;146(4):657-70. doi: 10.1111/imm.12533. Epub 2015 Oct 28. Immunology. 2015. PMID: 26370005 Free PMC article.
-
CpG-A oligonucleotides induce a monocyte-derived dendritic cell-like phenotype that preferentially activates CD8 T cells.J Immunol. 2003 Apr 1;170(7):3468-77. doi: 10.4049/jimmunol.170.7.3468. J Immunol. 2003. PMID: 12646607
-
Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory.Immunity. 2008 Feb;28(2):258-70. doi: 10.1016/j.immuni.2007.12.016. Immunity. 2008. PMID: 18275834
-
T cell activation pathways: B7, LFA-3, and ICAM-1 shape unique T cell profiles.Crit Rev Immunol. 1995;15(3-4):235-53. doi: 10.1615/critrevimmunol.v15.i3-4.30. Crit Rev Immunol. 1995. PMID: 8834450 Review.
-
T Cell Activation Pathways: B7, LFA-3, and ICAM-1 Shape Unique T Cell Profiles.Crit Rev Immunol. 2017;37(2-6):463-481. doi: 10.1615/CritRevImmunol.v37.i2-6.130. Crit Rev Immunol. 2017. PMID: 29773030 Review.
Cited by
-
Tumor-intrinsic role of ICAM-1 in driving metastatic progression of triple-negative breast cancer through direct interaction with EGFR.Mol Cancer. 2024 Oct 16;23(1):230. doi: 10.1186/s12943-024-02150-4. Mol Cancer. 2024. PMID: 39415210 Free PMC article.
-
Single-Cell RNA-Sequencing Reveals Interactions between Endometrial Stromal Cells, Epithelial Cells, and Lymphocytes during Mouse Embryo Implantation.Int J Mol Sci. 2022 Dec 22;24(1):213. doi: 10.3390/ijms24010213. Int J Mol Sci. 2022. PMID: 36613656 Free PMC article.
-
LFA-1 in T cell priming, differentiation, and effector functions.Trends Immunol. 2021 Aug;42(8):706-722. doi: 10.1016/j.it.2021.06.004. Epub 2021 Jul 12. Trends Immunol. 2021. PMID: 34266767 Free PMC article. Review.
-
Tonic 4-1BB Costimulation in Chimeric Antigen Receptors Impedes T Cell Survival and Is Vector-Dependent.Cell Rep. 2017 Oct 3;21(1):17-26. doi: 10.1016/j.celrep.2017.09.015. Cell Rep. 2017. PMID: 28978471 Free PMC article.
-
LFA-1 in T Cell Migration and Differentiation.Front Immunol. 2018 May 3;9:952. doi: 10.3389/fimmu.2018.00952. eCollection 2018. Front Immunol. 2018. PMID: 29774029 Free PMC article. Review.
References
-
- Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 2006;211:81–92. - PubMed
-
- Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J. Immunol. 2005;174:4465–4469. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

