Indometacin ameliorates high glucose-induced proliferation and invasion via modulation of e-cadherin in pancreatic cancer cells
- PMID: 23992308
- PMCID: PMC4085328
- DOI: 10.2174/09298673113209990249
Indometacin ameliorates high glucose-induced proliferation and invasion via modulation of e-cadherin in pancreatic cancer cells
Abstract
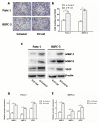
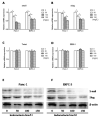
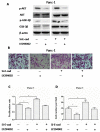
Indometacin, an inhibitor of cyclooxygenase-2 (COX-2), has been shown to exert anticancer effects in a variety of cancers. However, the effect and mechanism of indometacin on high glucose (HG)-induced proliferation and invasion of pancreatic cancer (PC) cells remain unclear. Multiple lines of evidence suggest that a large portion of pancreatic cancer (PC) patients suffer from either diabetes or HG which contributing PC progression. In this study, we report that indometacin down-regulated HG-induced proliferation and invasion via up-regulating E-cadherin but not COX-2 in PC cells. Additionally, the E-cadherin transcriptional repressors, Snail and Slug, were also involved in the process. Furthermore, the proliferation and invasion of PC cells, incubated in HG medium and treated with indometacin were significantly increased when E-cadherin was knocked down (Si-E-cad). Moreover, the protein levels of MMP-2, MMP-9, and VEGF were increased in PC cells transfected with Si-E-cad. Finally, the activation of the PI3K/AKT/GSK-3β signaling pathway was demonstrated to be involved in indometacin reversing HG-induced cell proliferation and invasion in PC cells. In conclusion, these results suggest that indometacin plays a key role in down-regulating HG-induced proliferation and invasion in PC cells. Our findings indicate that indometacin could be used as a novel therapeutic strategy to treat PC patients who simultaneously suffer from diabetes or HG.
Figures
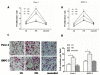
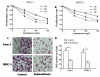
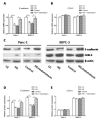
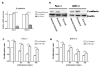
Similar articles
-
BMP2 accelerates the motility and invasiveness of gastric cancer cells via activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway.Exp Cell Res. 2010 Jan 1;316(1):24-37. doi: 10.1016/j.yexcr.2009.10.010. Epub 2009 Oct 14. Exp Cell Res. 2010. PMID: 19835871
-
Troglitazone ameliorates high glucose-induced EMT and dysfunction of SGLTs through PI3K/Akt, GSK-3β, Snail1, and β-catenin in renal proximal tubule cells.Am J Physiol Renal Physiol. 2010 May;298(5):F1263-75. doi: 10.1152/ajprenal.00475.2009. Epub 2009 Dec 16. Am J Physiol Renal Physiol. 2010. PMID: 20015942
-
ERalpha signaling through slug regulates E-cadherin and EMT.Oncogene. 2010 Mar 11;29(10):1451-62. doi: 10.1038/onc.2009.433. Epub 2010 Jan 18. Oncogene. 2010. PMID: 20101232
-
Elevated Expression of Zinc Finger Protein 703 Promotes Cell Proliferation and Metastasis through PI3K/AKT/GSK-3β Signalling in Oral Squamous Cell Carcinoma.Cell Physiol Biochem. 2017;44(3):920-934. doi: 10.1159/000485360. Epub 2017 Nov 24. Cell Physiol Biochem. 2017. Retraction in: Cell Physiol Biochem. 2021;55(1):138. doi: 10.33594/000000347 PMID: 29176314 Retracted.
-
Glucose metabolism and tumour microenvironment in pancreatic cancer: A key link in cancer progression.Front Immunol. 2022 Dec 12;13:1038650. doi: 10.3389/fimmu.2022.1038650. eCollection 2022. Front Immunol. 2022. PMID: 36578477 Free PMC article. Review.
Cited by
-
Pancreatic Cancer and Microenvironments: Implications of Anesthesia.Cancers (Basel). 2022 May 28;14(11):2684. doi: 10.3390/cancers14112684. Cancers (Basel). 2022. PMID: 35681664 Free PMC article. Review.
-
Glucose insult elicits hyperactivation of cancer stem cells through miR-424-cdc42-prdm14 signalling axis.Br J Cancer. 2017 Nov 21;117(11):1665-1675. doi: 10.1038/bjc.2017.335. Epub 2017 Oct 12. Br J Cancer. 2017. PMID: 29024936 Free PMC article.
-
Glucose promotes cell proliferation, glucose uptake and invasion in endometrial cancer cells via AMPK/mTOR/S6 and MAPK signaling.Gynecol Oncol. 2015 Sep;138(3):668-75. doi: 10.1016/j.ygyno.2015.06.036. Epub 2015 Jun 30. Gynecol Oncol. 2015. PMID: 26135947 Free PMC article.
-
High Glucose Concentrations Negatively Regulate the IGF1R/Src/ERK Axis through the MicroRNA-9 in Colorectal Cancer.Cells. 2019 Apr 8;8(4):326. doi: 10.3390/cells8040326. Cells. 2019. PMID: 30965609 Free PMC article.
-
TRIM22 confers poor prognosis and promotes epithelial-mesenchymal transition through regulation of AKT/GSK3β/β-catenin signaling in non-small cell lung cancer.Oncotarget. 2017 Jul 1;8(37):62069-62080. doi: 10.18632/oncotarget.18911. eCollection 2017 Sep 22. Oncotarget. 2017. PMID: 28977927 Free PMC article.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J. Clin. 2013;63(1):11–30. - PubMed
-
- Morrison M. Pancreatic cancer and diabetes. Adv. Exp. Med. Biol. 2012;771:229–239. - PubMed
-
- Slim I, Ach K, Chefii R, Trimech-Ajmi S, Landolsi A, Chadli-Chaieb M, Maaroufi-Beizig A, Chaieb L. Diabetes mellitus as an early symptom of pancreatic cancer diagnosed three years later. Ann. Endocrinol. 2009;70(1):76–79. - PubMed
-
- Hwang A, Narayan V, Yang YX. Type 2 diabetes mellitus and survival in pancreatic adenocarcinoma: a retrospective cohort study. Cancer. 2013;119(2):404–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

