mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation
- PMID: 23980067
- PMCID: PMC3790516
- DOI: 10.1182/blood-2013-04-495119
mRNA-engineered mesenchymal stem cells for targeted delivery of interleukin-10 to sites of inflammation
Abstract
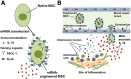
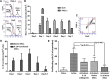
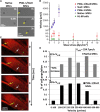
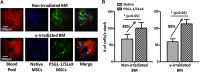
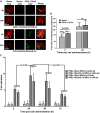
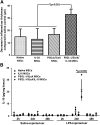
Mesenchymal stem cells (MSCs) are promising candidates for cell-based therapy to treat several diseases and are compelling to consider as vehicles for delivery of biological agents. However, MSCs appear to act through a seemingly limited "hit-and-run" mode to quickly exert their therapeutic impact, mediated by several mechanisms, including a potent immunomodulatory secretome. Furthermore, MSC immunomodulatory properties are highly variable and the secretome composition following infusion is uncertain. To determine whether a transiently controlled antiinflammatory MSC secretome could be achieved at target sites of inflammation, we harnessed mRNA transfection to generate MSCs that simultaneously express functional rolling machinery (P-selectin glycoprotein ligand-1 [PSGL-1] and Sialyl-Lewis(x) [SLeX]) to rapidly target inflamed tissues and that express the potent immunosuppressive cytokine interleukin-10 (IL-10), which is not inherently produced by MSCs. Indeed, triple-transfected PSGL-1/SLeX/IL-10 MSCs transiently increased levels of IL-10 in the inflamed ear and showed a superior antiinflammatory effect in vivo, significantly reducing local inflammation following systemic administration. This was dependent on rapid localization of MSCs to the inflamed site. Overall, this study demonstrates that despite the rapid clearance of MSCs in vivo, engineered MSCs can be harnessed via a "hit-and-run" action for the targeted delivery of potent immunomodulatory factors to treat distant sites of inflammation.
Figures
Similar articles
-
Mesenchymal stem cells engineered to express selectin ligands and IL-10 exert enhanced therapeutic efficacy in murine experimental autoimmune encephalomyelitis.Biomaterials. 2016 Jan;77:87-97. doi: 10.1016/j.biomaterials.2015.11.005. Epub 2015 Nov 10. Biomaterials. 2016. PMID: 26584349 Free PMC article.
-
Transplantation of mesenchymal stem cells genetically engineered to overexpress interleukin-10 promotes alternative inflammatory response in rat model of traumatic brain injury.J Neuroinflammation. 2019 Jan 5;16(1):2. doi: 10.1186/s12974-018-1383-2. J Neuroinflammation. 2019. PMID: 30611291 Free PMC article.
-
Combinatorial targeting of cancer bone metastasis using mRNA engineered stem cells.EBioMedicine. 2019 Jul;45:39-57. doi: 10.1016/j.ebiom.2019.06.047. Epub 2019 Jul 4. EBioMedicine. 2019. PMID: 31281099 Free PMC article.
-
Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-conditioning.Front Immunol. 2018 Dec 4;9:2837. doi: 10.3389/fimmu.2018.02837. eCollection 2018. Front Immunol. 2018. PMID: 30564236 Free PMC article. Review.
-
Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome.Cells. 2019 May 16;8(5):467. doi: 10.3390/cells8050467. Cells. 2019. PMID: 31100966 Free PMC article. Review.
Cited by
-
Biosimilar Gene Therapy: Investigational Assessment of Secukinumab Gene Therapy.Cell J. 2020 Jan;21(4):433-443. doi: 10.22074/cellj.2020.6309. Epub 2019 Jul 29. Cell J. 2020. PMID: 31376325 Free PMC article.
-
Iron oxide nanoparticles promote the migration of mesenchymal stem cells to injury sites.Int J Nanomedicine. 2019 Jan 14;14:573-589. doi: 10.2147/IJN.S184920. eCollection 2019. Int J Nanomedicine. 2019. PMID: 30666115 Free PMC article.
-
The Immunomodulatory Properties of Amniotic Cells: The Two Sides of the Coin.Cell Transplant. 2018 Jan;27(1):31-44. doi: 10.1177/0963689717742819. Cell Transplant. 2018. PMID: 29562786 Free PMC article.
-
A Comparison of Immune Responses Exerted Following Syngeneic, Allogeneic, and Xenogeneic Transplantation of Mesenchymal Stem Cells into the Mouse Brain.Int J Mol Sci. 2020 Apr 26;21(9):3052. doi: 10.3390/ijms21093052. Int J Mol Sci. 2020. PMID: 32357509 Free PMC article.
-
The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy.Stem Cell Res Ther. 2015 Dec 1;6:234. doi: 10.1186/s13287-015-0240-9. Stem Cell Res Ther. 2015. PMID: 26620426 Free PMC article. Review.
References
-
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. - PubMed
-
- Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–478. - PubMed
-
- Hoogduijn MJ, Popp F, Verbeek R, et al. The immunomodulatory properties of mesenchymal stem cells and their use for immunotherapy. Int Immunopharmacol. 2010;10(12):1496–1500. - PubMed
-
- Liang J, Zhang H, Hua B. Allogeneic mesenchymal stem cells transplantation in treatment of multiple sclerosis. Mult Scler. 2009;15(5):644–646. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

