Cross talk between the Akt and p38α pathways in macrophages downstream of Toll-like receptor signaling
- PMID: 23979601
- PMCID: PMC3811899
- DOI: 10.1128/MCB.01691-12
Cross talk between the Akt and p38α pathways in macrophages downstream of Toll-like receptor signaling
Abstract
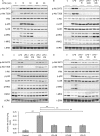
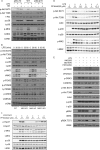
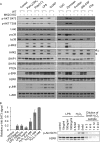
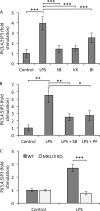
The stimulation of Toll-like receptors (TLRs) on macrophages by pathogen-associated molecular patterns (PAMPs) results in the activation of intracellular signaling pathways that are required for initiating a host immune response. Both phosphatidylinositol 3-kinase (PI3K)-Akt and p38 mitogen-activated protein kinase (MAPK) signaling pathways are activated rapidly in response to TLR activation and are required to coordinate effective host responses to pathogen invasion. In this study, we analyzed the role of the p38-dependent kinases MK2/3 in the activation of Akt and show that lipopolysaccharide (LPS)-induced phosphorylation of Akt on Thr308 and Ser473 requires p38α and MK2/3. In cells treated with p38 inhibitors or an MK2/3 inhibitor, phosphorylation of Akt on Ser473 and Thr308 is reduced and Akt activity is inhibited. Furthermore, BMDMs deficient in MK2/3 display greatly reduced phosphorylation of Ser473 and Thr308 following TLR stimulation. However, MK2/3 do not directly phosphorylate Akt in macrophages but act upstream of PDK1 and mTORC2 to regulate Akt phosphorylation. Akt is recruited to phosphatidylinositol 3,4,5-trisphosphate (PIP3) in the membrane, where it is activated by PDK1 and mTORC2. Analysis of lipid levels in MK2/3-deficient bone marrow-derived macrophages (BMDMs) revealed a role for MK2/3 in regulating Akt activity by affecting availability of PIP3 at the membrane. These data describe a novel role for p38α-MK2/3 in regulating TLR-induced Akt activation in macrophages.
Figures


Similar articles
-
Microglial p38α MAPK is a key regulator of proinflammatory cytokine up-regulation induced by toll-like receptor (TLR) ligands or beta-amyloid (Aβ).J Neuroinflammation. 2011 Jul 6;8:79. doi: 10.1186/1742-2094-8-79. J Neuroinflammation. 2011. PMID: 21733175 Free PMC article.
-
p38 Kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils.J Biol Chem. 2001 Feb 2;276(5):3517-23. doi: 10.1074/jbc.M005953200. Epub 2000 Oct 20. J Biol Chem. 2001. PMID: 11042204
-
MAPK-activated protein kinase-2 (MK2)-mediated formation and phosphorylation-regulated dissociation of the signal complex consisting of p38, MK2, Akt, and Hsp27.J Biol Chem. 2006 Dec 1;281(48):37215-26. doi: 10.1074/jbc.M603622200. Epub 2006 Oct 2. J Biol Chem. 2006. PMID: 17015449
-
Targeting the p38α pathway in chronic inflammatory diseases: Could activation, not inhibition, be the appropriate therapeutic strategy?Pharmacol Ther. 2022 Jul;235:108153. doi: 10.1016/j.pharmthera.2022.108153. Epub 2022 Feb 1. Pharmacol Ther. 2022. PMID: 35121002 Review.
-
Charting protein dephosphorylation triggered by Toll-like receptor (TLR) signaling in macrophages and its role in health and disease.Int Rev Cell Mol Biol. 2021;361:211-243. doi: 10.1016/bs.ircmb.2021.02.003. Epub 2021 Mar 2. Int Rev Cell Mol Biol. 2021. PMID: 34074495 Review.
Cited by
-
Characterization of small HSPs from Anemonia viridis reveals insights into molecular evolution of alpha crystallin genes among cnidarians.PLoS One. 2014 Sep 24;9(9):e105908. doi: 10.1371/journal.pone.0105908. eCollection 2014. PLoS One. 2014. PMID: 25251681 Free PMC article.
-
Immune modulating capability of two exopolysaccharide-producing Bifidobacterium strains in a Wistar rat model.Biomed Res Int. 2014;2014:106290. doi: 10.1155/2014/106290. Epub 2014 May 29. Biomed Res Int. 2014. PMID: 24971309 Free PMC article.
-
Structural and functional basis for p38-MK2-activated Rsk signaling in toll-like receptor-stimulated dendritic cells.Mol Cell Biol. 2015 Jan;35(1):132-40. doi: 10.1128/MCB.00773-14. Epub 2014 Oct 20. Mol Cell Biol. 2015. PMID: 25332232 Free PMC article.
-
Dual Effect of Soloxolone Methyl on LPS-Induced Inflammation In Vitro and In Vivo.Int J Mol Sci. 2020 Oct 23;21(21):7876. doi: 10.3390/ijms21217876. Int J Mol Sci. 2020. PMID: 33114200 Free PMC article.
-
The TLR and IL-1 signalling network at a glance.J Cell Sci. 2014 Jun 1;127(Pt 11):2383-90. doi: 10.1242/jcs.149831. Epub 2014 May 14. J Cell Sci. 2014. PMID: 24829146 Free PMC article. Review.
References
-
- Leevers SJ, Vanhaesebroeck B, Waterfield MD. 1999. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr. Opin. Cell Biol. 11:219–225 - PubMed
-
- Franke TF. 2008. PI3K/Akt: getting it right matters. Oncogene 27:6473–6488 - PubMed
-
- Hazeki K, Nigorikawa K, Hazeki O. 2007. Role of phosphoinositide 3-kinase in innate immunity. Biol. Pharm. Bull. 30:1617–1623 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
