A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia
- PMID: 23979158
- PMCID: PMC4381282
- DOI: 10.1172/JCI70026
A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia
Abstract
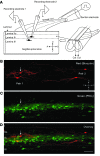
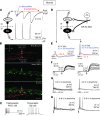
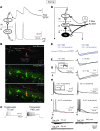
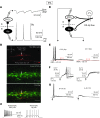
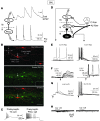
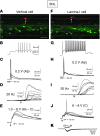
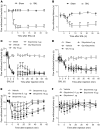
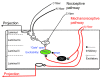
Neuropathic pain is characterized by mechanical allodynia induced by low-threshold myelinated Aβ-fiber activation. The original gate theory of pain proposes that inhibitory interneurons in the lamina II of the spinal dorsal horn (DH) act as "gate control" units for preventing the interaction between innocuous and nociceptive signals. However, our understanding of the neuronal circuits underlying pain signaling and modulation in the spinal DH is incomplete. Using a rat model, we have shown that the convergence of glycinergic inhibitory and excitatory Aβ-fiber inputs onto PKCγ+ neurons in the superficial DH forms a feed-forward inhibitory circuit that prevents Aβ input from activating the nociceptive pathway. This feed-forward inhibition was suppressed following peripheral nerve injury or glycine blockage, leading to inappropriate induction of action potential outputs in the nociceptive pathway by Aβ-fiber stimulation. Furthermore, spinal blockage of glycinergic synaptic transmission in vivo induced marked mechanical allodynia. Our findings identify a glycinergic feed-forward inhibitory circuit that functions as a gate control to separate the innocuous mechanoreceptive pathway and the nociceptive pathway in the spinal DH. Disruption of this glycinergic inhibitory circuit after peripheral nerve injury has the potential to elicit mechanical allodynia, a cardinal symptom of neuropathic pain.
Figures
Similar articles
-
Effects of activation of group III metabotropic glutamate receptors on spinal synaptic transmission in a rat model of neuropathic pain.Neuroscience. 2009 Jan 23;158(2):875-84. doi: 10.1016/j.neuroscience.2008.10.042. Epub 2008 Oct 30. Neuroscience. 2009. PMID: 19017536 Free PMC article.
-
Activation of medullary dorsal horn γ isoform of protein kinase C interneurons is essential to the development of both static and dynamic facial mechanical allodynia.Eur J Neurosci. 2016 Mar;43(6):802-10. doi: 10.1111/ejn.13165. Epub 2016 Feb 9. Eur J Neurosci. 2016. PMID: 26750151
-
Synaptic Dynamics of the Feed-forward Inhibitory Circuitry Gating Mechanical Allodynia in Mice.Anesthesiology. 2020 May;132(5):1212-1228. doi: 10.1097/ALN.0000000000003194. Anesthesiology. 2020. PMID: 32101975
-
PKCγ interneurons, a gateway to pathological pain in the dorsal horn.J Neural Transm (Vienna). 2020 Apr;127(4):527-540. doi: 10.1007/s00702-020-02162-6. Epub 2020 Feb 27. J Neural Transm (Vienna). 2020. PMID: 32108249 Review.
-
Inhibition of Glycine Re-Uptake: A Potential Approach for Treating Pain by Augmenting Glycine-Mediated Spinal Neurotransmission and Blunting Central Nociceptive Signaling.Biomolecules. 2021 Jun 10;11(6):864. doi: 10.3390/biom11060864. Biomolecules. 2021. PMID: 34200954 Free PMC article. Review.
Cited by
-
Computational modeling of peripheral pain: a commentary.Biomed Eng Online. 2015 Jun 11;14:56. doi: 10.1186/s12938-015-0049-x. Biomed Eng Online. 2015. PMID: 26062616 Free PMC article. Review.
-
Glycine Receptor Subtypes and Their Roles in Nociception and Chronic Pain.Front Mol Neurosci. 2022 Mar 23;15:848642. doi: 10.3389/fnmol.2022.848642. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35401105 Free PMC article. Review.
-
The T-Type Calcium Channel Cav3.2 in Somatostatin Interneurons in Spinal Dorsal Horn Participates in Mechanosensation and Mechanical Allodynia in Mice.Front Cell Neurosci. 2022 Apr 8;16:875726. doi: 10.3389/fncel.2022.875726. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35465611 Free PMC article.
-
Pathological Mechanisms and Therapeutic Targets for Trigeminal Neuropathic Pain.Medicines (Basel). 2019 Aug 22;6(3):91. doi: 10.3390/medicines6030091. Medicines (Basel). 2019. PMID: 31443547 Free PMC article. Review.
-
Functional and anatomical analyses of active spinal circuits in a mouse model of chronic pain.Pain. 2024 Mar 1;165(3):685-697. doi: 10.1097/j.pain.0000000000003068. Epub 2023 Oct 10. Pain. 2024. PMID: 37820238 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

