Berberine suppresses gero-conversion from cell cycle arrest to senescence
- PMID: 23974852
- PMCID: PMC3796215
- DOI: 10.18632/aging.100593
Berberine suppresses gero-conversion from cell cycle arrest to senescence
Abstract
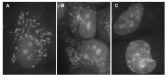
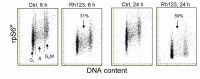
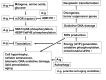
Berberine (BRB), a natural alkaloid, has a long history of medicinal use in both Ayurvedic and old Chinese medicine. Recently, available as a dietary supplement, Berberine is reported to have application in treatment of variety diseases. Previously we observed that BRB inhibited mTOR/S6 signaling concurrently with reduction of the level of endogenous oxidants and constitutive DNA damage response. We currently tested whether Berberine can affect premature, stress-induced cellular senescence caused by mitoxantrone. The depth of senescence was quantitatively measured by morphometric parameters, senescence-associated β-galactosidase, induction of p21WAF1, replication stress (γH2AX expression), and mTOR signaling; the latter revealed by ribosomal S6 protein (rpS6) phosphorylation. All these markers of senescence were distinctly diminished, in a concentration-dependent manner, by Berberine. In view of the evidence that BRB localizes in mitochondria, inhibits respiratory electron chain and activates AMPK, the observed attenuation of the replication stress-induced cellular senescence most likely is mediated by AMPK that leads to inhibition of mTOR signaling. In support of this mechanism is the observation that rhodamine123, the cationic probe targeting mitochondrial electron chain, also suppressed rpS6 phosphorylation. The present findings reveal that: (a) in cells induced to senescence BRB exhibits gero-suppressive properties by means of mTOR/S6 inhibition; (b) in parallel, BRB reduces the level of constitutive DNA damage response, previously shown to report oxidative DNA damage by endogenous ROS; (c) there appears to a causal linkage between the (a) and (b) activities; (d) the in vitro model of premature stress-induced senescence can be used to assess effectiveness of potential gero-suppressive agents targeting mTOR/S6 and ROS signaling; (e) since most of the reported beneficial effects of BRB are in age-relate diseases, it is likely that gero-suppression is the primary activity of this traditional medicine.
Conflict of interest statement
The authors have no conflicting interest to declare.
Figures




Similar articles
-
Potential anti-aging agents suppress the level of constitutive mTOR- and DNA damage- signaling.Aging (Albany NY). 2012 Dec;4(12):952-65. doi: 10.18632/aging.100521. Aging (Albany NY). 2012. PMID: 23363784 Free PMC article.
-
In search of antiaging modalities: evaluation of mTOR- and ROS/DNA damage-signaling by cytometry.Cytometry A. 2014 May;85(5):386-99. doi: 10.1002/cyto.a.22452. Epub 2014 Feb 22. Cytometry A. 2014. PMID: 24677687 Free PMC article. Review.
-
Attenuation of replication stress-induced premature cellular senescence to assess anti-aging modalities.Curr Protoc Cytom. 2014 Jul 1;69:9.47.1-9.47.10. doi: 10.1002/0471142956.cy0947s69. Curr Protoc Cytom. 2014. PMID: 24984966 Free PMC article.
-
1,8-Cineole promotes G0/G1 cell cycle arrest and oxidative stress-induced senescence in HepG2 cells and sensitizes cells to anti-senescence drugs.Life Sci. 2020 Feb 15;243:117271. doi: 10.1016/j.lfs.2020.117271. Epub 2020 Jan 8. Life Sci. 2020. PMID: 31926243
-
Rapamycin and the inhibition of the secretory phenotype.Exp Gerontol. 2017 Aug;94:89-92. doi: 10.1016/j.exger.2017.01.026. Epub 2017 Feb 4. Exp Gerontol. 2017. PMID: 28167236 Review.
Cited by
-
Tumor promoter-induced cellular senescence: cell cycle arrest followed by geroconversion.Oncotarget. 2014 Dec 30;5(24):12715-27. doi: 10.18632/oncotarget.3011. Oncotarget. 2014. PMID: 25587030 Free PMC article.
-
Rhizoma Coptidis and Berberine as a Natural Drug to Combat Aging and Aging-Related Diseases via Anti-Oxidation and AMPK Activation.Aging Dis. 2017 Dec 1;8(6):760-777. doi: 10.14336/AD.2016.0620. eCollection 2017 Dec. Aging Dis. 2017. PMID: 29344415 Free PMC article. Review.
-
Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs.Aging (Albany NY). 2017 Jun 12;9(6):1477-1536. doi: 10.18632/aging.101250. Aging (Albany NY). 2017. PMID: 28611316 Free PMC article. Review.
-
Berberine attenuates brain aging via stabilizing redox homeostasis and inflammation in an accelerated senescence model of Wistar rats.Metab Brain Dis. 2024 Jun;39(5):649-659. doi: 10.1007/s11011-024-01350-7. Epub 2024 May 10. Metab Brain Dis. 2024. PMID: 38727934
-
The Preconditioning of Berberine Suppresses Hydrogen Peroxide-Induced Premature Senescence via Regulation of Sirtuin 1.Oxid Med Cell Longev. 2017;2017:2391820. doi: 10.1155/2017/2391820. Epub 2017 Jul 2. Oxid Med Cell Longev. 2017. PMID: 28751929 Free PMC article.
References
-
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. - PubMed
-
- Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. - PubMed
-
- Sherr CJ, DePinho RA. Cellular senescence: Mitotic clock or culture shock? Cell. 2000;102:407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

