Inflammatory cytokines induce a unique mineralizing phenotype in mesenchymal stem cells derived from human bone marrow
- PMID: 23970554
- PMCID: PMC3795248
- DOI: 10.1074/jbc.M113.471268
Inflammatory cytokines induce a unique mineralizing phenotype in mesenchymal stem cells derived from human bone marrow
Abstract
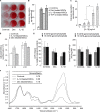
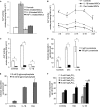
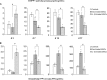
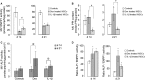
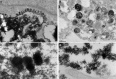
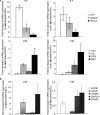
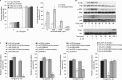
Bone marrow contains mesenchymal stem cells (MSCs) that can differentiate along multiple mesenchymal lineages. In this capacity they are thought to be important in the intrinsic turnover and repair of connective tissues while also serving as a basis for tissue engineering and regenerative medicine. However, little is known of the biological responses of human MSCs to inflammatory conditions. When cultured with IL-1β, marrow-derived MSCs from 8 of 10 human subjects deposited copious hydroxyapatite, in which authenticity was confirmed by Fourier transform infrared spectroscopy. Transmission electron microscopy revealed the production of fine needles of hydroxyapatite in conjunction with matrix vesicles. Alkaline phosphatase activity did not increase in response to inflammatory mediators, but PPi production fell, reflecting lower ectonucleotide pyrophosphatase activity in cells and matrix vesicles. Because PPi is the major physiological inhibitor of mineralization, its decline generated permissive conditions for hydroxyapatite formation. This is in contrast to MSCs treated with dexamethasone, where PPi levels did not fall and mineralization was fuelled by a large and rapid increase in alkaline phosphatase activity. Bone sialoprotein was the only osteoblast marker strongly induced by IL-1β; thus these cells do not become osteoblasts despite depositing abundant mineral. RT-PCR did not detect transcripts indicative of alternative mesenchymal lineages, including chondrocytes, myoblasts, adipocytes, ligament, tendon, or vascular smooth muscle cells. IL-1β phosphorylated multiple MAPKs and activated nuclear factor-κB (NF-κB). Certain inhibitors of MAPK and PI3K, but not NF-κB, prevented mineralization. The findings are of importance to soft tissue mineralization, tissue engineering, and regenerative medicine.
Keywords: Biomineralization; Bone; Cell Differentiation; Inflammation; Interleukin; Tissue Engineering.
Figures
Similar articles
-
Inhibitory effect of alcohol on osteogenic differentiation in human bone marrow-derived mesenchymal stem cells.Alcohol Clin Exp Res. 2004 Mar;28(3):468-79. doi: 10.1097/01.alc.0000118315.58404.c1. Alcohol Clin Exp Res. 2004. PMID: 15084905
-
Bioinspired mineralization of a functionalized injectable dense collagen hydrogel through silk sericin incorporation.Biomater Sci. 2019 Feb 26;7(3):1064-1077. doi: 10.1039/c8bm01060a. Biomater Sci. 2019. PMID: 30629053
-
Symphytum officinale augments osteogenesis in human bone marrow-derived mesenchymal stem cells in vitro as they differentiate into osteoblasts.J Ethnopharmacol. 2020 Feb 10;248:112329. doi: 10.1016/j.jep.2019.112329. Epub 2019 Oct 28. J Ethnopharmacol. 2020. PMID: 31672526
-
Exposure to pro-inflammatory cytokines upregulates MMP-9 synthesis by mesenchymal stem cells-derived osteoprogenitors.Histochem Cell Biol. 2008 May;129(5):589-97. doi: 10.1007/s00418-008-0391-1. Epub 2008 Feb 15. Histochem Cell Biol. 2008. PMID: 18274772
-
Chronic inflammation induced by microneedling and the use of bone marrow stem cell cytokines.J Tissue Viability. 2022 Nov;31(4):687-692. doi: 10.1016/j.jtv.2022.08.001. Epub 2022 Sep 2. J Tissue Viability. 2022. PMID: 36115793 Review.
Cited by
-
Role of autophagy in tumor necrosis factor-α-induced apoptosis of osteoblast cells.J Investig Med. 2017 Aug;65(6):1014-1020. doi: 10.1136/jim-2017-000426. Epub 2017 Jun 20. J Investig Med. 2017. PMID: 28634253 Free PMC article.
-
Analysis of the Pro- and Anti-Inflammatory Cytokines Secreted by Adult Stem Cells during Differentiation.Stem Cells Int. 2015;2015:412467. doi: 10.1155/2015/412467. Epub 2015 Aug 2. Stem Cells Int. 2015. PMID: 26300921 Free PMC article.
-
β-catenin signaling induces the osteoblastogenic differentiation of human pre-osteoblastic and bone marrow stromal cells mainly through the upregulation of osterix expression.Int J Mol Med. 2015 Dec;36(6):1572-82. doi: 10.3892/ijmm.2015.2382. Epub 2015 Oct 20. Int J Mol Med. 2015. PMID: 26496941 Free PMC article.
-
Alterations of tendons in diabetes mellitus: what are the current findings?Int Orthop. 2015 Aug;39(8):1465-73. doi: 10.1007/s00264-015-2775-x. Epub 2015 May 6. Int Orthop. 2015. PMID: 25944078 Review.
-
Opposing TNF-α/IL-1β- and BMP-2-activated MAPK signaling pathways converge on Runx2 to regulate BMP-2-induced osteoblastic differentiation.Cell Death Dis. 2014 Apr 17;5(4):e1187. doi: 10.1038/cddis.2014.101. Cell Death Dis. 2014. PMID: 24743742 Free PMC article.
References
-
- Caplan A. I., Dennis J. E. (2006) Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 98, 1076–1084 - PubMed
-
- Arthur A., Zannettino A., Gronthos S. (2009) The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair. J. Cell. Physiol. 218, 237–245 - PubMed
-
- Pape H. C., Marcucio R., Humphrey C., Colnot C., Knobe M., Harvey E. J. (2010) Trauma-induced inflammation and fracture healing. J. Orthop. Trauma 24, 522–525 - PubMed
-
- Bastian O., Pillay J., Alblas J., Leenen L., Koenderman L., Blokhuis T. (2011) Systemic inflammation and fracture healing. J. Leukoc. Biol. 89, 669–673 - PubMed
-
- Ni Choileain N., Redmond H. P. (2006) Cell response to surgery. Arch. Surg. 141, 1132–1140 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

