Emergence of polyclonal FLT3 tyrosine kinase domain mutations during sequential therapy with sorafenib and sunitinib in FLT3-ITD-positive acute myeloid leukemia
- PMID: 23969938
- PMCID: PMC3878304
- DOI: 10.1158/1078-0432.CCR-13-1323
Emergence of polyclonal FLT3 tyrosine kinase domain mutations during sequential therapy with sorafenib and sunitinib in FLT3-ITD-positive acute myeloid leukemia
Abstract
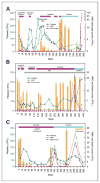
Purpose: To evaluate the clinical activity of sequential therapy with sorafenib and sunitinib in FMS-like tyrosine kinase 3 (FLT3)-internal tandem duplication (ITD)-positive acute myelogenous leukemia (AML) and monitor the emergence of secondary FLT3 tyrosine kinase domain (TKD) mutations during treatment.
Experimental design: Six children with relapsed/refractory AML were treated with sorafenib in combination with clofarabine and cytarabine, followed by single-agent sorafenib if not a candidate for transplantation. Sunitinib was initiated after sorafenib relapse. Bone marrow samples were obtained for assessment of FLT3 TKD mutations by deep amplicon sequencing. The phase of secondary mutations with ITD alleles was assessed by cloning and sequencing of FLT3 exons 14 through 20. Identified mutations were modeled in Ba/F3 cells, and the effect of kinase inhibitors on FLT3 signaling and cell viability was assessed.
Results: Four patients achieved complete remission, but 3 receiving maintenance therapy with sorafenib relapsed after 14 to 37 weeks. Sunitinib reduced circulating blasts in two patients and marrow blasts in one. Two patients did not respond to sorafenib combination therapy or sunitinib. FLT3 mutations at residues D835 and F691 were observed in sorafenib resistance samples on both ITD-positive and -negative alleles. Deep sequencing revealed low-level mutations and their evolution during sorafenib treatment. Sunitinib suppressed leukemic clones with D835H and F691L mutations, but not D835Y. Cells expressing sorafenib-resistant FLT3 mutations were sensitive to sunitinib in vitro.
Conclusions: Sunitinib has activity in patients that are resistant to sorafenib and harbor secondary FLT3 TKD mutations. The use of sensitive methods to monitor FLT3 mutations during therapy may allow individualized treatment with the currently available kinase inhibitors.
©2013 AACR.
Conflict of interest statement
The authors declared no conflict of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Figures


Similar articles
-
Reversal of acquired drug resistance in FLT3-mutated acute myeloid leukemia cells via distinct drug combination strategies.Clin Cancer Res. 2014 May 1;20(9):2363-74. doi: 10.1158/1078-0432.CCR-13-2052. Epub 2014 Mar 11. Clin Cancer Res. 2014. PMID: 24619500 Free PMC article.
-
Sensitivity toward sorafenib and sunitinib varies between different activating and drug-resistant FLT3-ITD mutations.Exp Hematol. 2007 Oct;35(10):1522-6. doi: 10.1016/j.exphem.2007.07.008. Exp Hematol. 2007. PMID: 17889720
-
Crenolanib is active against models of drug-resistant FLT3-ITD-positive acute myeloid leukemia.Blood. 2013 Nov 21;122(22):3607-15. doi: 10.1182/blood-2013-07-513044. Epub 2013 Sep 17. Blood. 2013. PMID: 24046014 Free PMC article.
-
FLT3 inhibitors in the treatment of acute myeloid leukemia: current status and future perspectives.Minerva Med. 2020 Oct;111(5):427-442. doi: 10.23736/S0026-4806.20.06989-X. Epub 2020 Sep 21. Minerva Med. 2020. PMID: 32955823 Review.
-
The role of small molecule Flt3 receptor protein-tyrosine kinase inhibitors in the treatment of Flt3-positive acute myelogenous leukemias.Pharmacol Res. 2020 May;155:104725. doi: 10.1016/j.phrs.2020.104725. Epub 2020 Feb 25. Pharmacol Res. 2020. PMID: 32109580 Review.
Cited by
-
Targeting Oncogenic Signaling in Mutant FLT3 Acute Myeloid Leukemia: The Path to Least Resistance.Int J Mol Sci. 2018 Oct 16;19(10):3198. doi: 10.3390/ijms19103198. Int J Mol Sci. 2018. PMID: 30332834 Free PMC article. Review.
-
Molecularly targeted drug combinations demonstrate selective effectiveness for myeloid- and lymphoid-derived hematologic malignancies.Proc Natl Acad Sci U S A. 2017 Sep 5;114(36):E7554-E7563. doi: 10.1073/pnas.1703094114. Epub 2017 Aug 7. Proc Natl Acad Sci U S A. 2017. PMID: 28784769 Free PMC article.
-
Gilteritinib is a clinically active FLT3 inhibitor with broad activity against FLT3 kinase domain mutations.Blood Adv. 2020 Feb 11;4(3):514-524. doi: 10.1182/bloodadvances.2019000919. Blood Adv. 2020. PMID: 32040554 Free PMC article.
-
The Current State of FLT3 Inhibition in Acute Myeloid Leukemia - Pitfalls and Promises.J Cell Signal (Los Angel). 2017;2(4):1000166. doi: 10.4172/2576-1471.1000166. Epub 2017 Oct 16. J Cell Signal (Los Angel). 2017. PMID: 29806049 Free PMC article. No abstract available.
-
Class III Receptor Tyrosine Kinases in Acute Leukemia - Biological Functions and Modern Laboratory Analysis.Biomark Insights. 2015 Aug 5;10(Suppl 3):1-14. doi: 10.4137/BMI.S22433. eCollection 2015. Biomark Insights. 2015. PMID: 26309392 Free PMC article. Review.
References
-
- Knapper S. The clinical development of FLT3 inhibitors in acute myeloid leukemia. Expert Opin Investig Drugs. 2011;20:1377–95. - PubMed
-
- Swords R, Freeman C, Giles F. Targeting the FMS-like tyrosine kinase 3 in acute myeloid leukemia. Leukemia. 2012;26:2176–85. - PubMed
-
- O’Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, et al. SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood. 2003;101:3597–605. - PubMed
-
- Zhang W, Konopleva M, Shi YX, McQueen T, Harris D, Ling X, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100:184–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

