O-GlcNAcylation: A New Cancer Hallmark?
- PMID: 23964270
- PMCID: PMC3740238
- DOI: 10.3389/fendo.2013.00099
O-GlcNAcylation: A New Cancer Hallmark?
Abstract
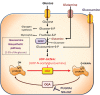
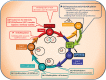
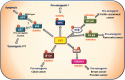
O-linked N-acetylglucosaminylation (O-GlcNAcylation) is a reversible post-translational modification consisting in the addition of a sugar moiety to serine/threonine residues of cytosolic or nuclear proteins. Catalyzed by O-GlcNAc-transferase (OGT) and removed by O-GlcNAcase, this dynamic modification is dependent on environmental glucose concentration. O-GlcNAcylation regulates the activities of a wide panel of proteins involved in almost all aspects of cell biology. As a nutrient sensor, O-GlcNAcylation can relay the effects of excessive nutritional intake, an important cancer risk factor, on protein activities and cellular functions. Indeed, O-GlcNAcylation has been shown to play a significant role in cancer development through different mechanisms. O-GlcNAcylation and OGT levels are increased in different cancers (breast, prostate, colon…) and vary during cell cycle progression. Modulating their expression or activity can alter cancer cell proliferation and/or invasion. Interestingly, major oncogenic factors have been shown to be directly O-GlcNAcylated (p53, MYC, NFκB, β-catenin…). Furthermore, chromatin dynamics is modulated by O-GlcNAc. DNA methylation enzymes of the Tet family, involved epigenetic alterations associated with cancer, were recently found to interact with and target OGT to multi-molecular chromatin-remodeling complexes. Consistently, histones are subjected to O-GlcNAc modifications which regulate their function. Increasing number of evidences point out the central involvement of O-GlcNAcylation in tumorigenesis, justifying the attention received as a potential new approach for cancer treatment. However, comprehension of the underlying mechanism remains at its beginnings. Future challenge will be to address directly the role of O-GlcNAc-modified residues in oncogenic-related proteins to eventually propose novel strategies to alter cancer development and/or progression.
Keywords: O-GlcNAc; O-glycosylation; cancer; cell cycle; epigenetics; metastasis; post-translational modification; transcription factors.
Figures
Similar articles
-
Roles of ten-eleven translocation family proteins and their O-linked β-N-acetylglucosaminylated forms in cancer development.Oncol Lett. 2021 Jan;21(1):1. doi: 10.3892/ol.2020.12262. Epub 2020 Nov 3. Oncol Lett. 2021. PMID: 33240407 Free PMC article. Review.
-
Feedback Regulation of O-GlcNAc Transferase through Translation Control to Maintain Intracellular O-GlcNAc Homeostasis.Int J Mol Sci. 2021 Mar 27;22(7):3463. doi: 10.3390/ijms22073463. Int J Mol Sci. 2021. PMID: 33801653 Free PMC article.
-
Undetectable histone O-GlcNAcylation in mammalian cells.Epigenetics. 2015;10(8):677-91. doi: 10.1080/15592294.2015.1060387. Epigenetics. 2015. PMID: 26075789 Free PMC article.
-
O-GlcNAcylation and O-GlcNAc Cycling Regulate Gene Transcription: Emerging Roles in Cancer.Cancers (Basel). 2021 Apr 1;13(7):1666. doi: 10.3390/cancers13071666. Cancers (Basel). 2021. PMID: 33916244 Free PMC article. Review.
-
Functional Analysis of O-GlcNAcylation in Cancer Metastasis.Front Oncol. 2020 Oct 27;10:585288. doi: 10.3389/fonc.2020.585288. eCollection 2020. Front Oncol. 2020. PMID: 33194731 Free PMC article. Review.
Cited by
-
Silencing the Nucleocytoplasmic O-GlcNAc Transferase Reduces Proliferation, Adhesion, and Migration of Cancer and Fetal Human Colon Cell Lines.Front Endocrinol (Lausanne). 2016 May 25;7:46. doi: 10.3389/fendo.2016.00046. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27252680 Free PMC article.
-
Inhibition of O-GlcNAc transferase activates type I interferon-dependent antitumor immunity by bridging cGAS-STING pathway.bioRxiv [Preprint]. 2024 Jun 11:2023.12.14.571787. doi: 10.1101/2023.12.14.571787. bioRxiv. 2024. Update in: Elife. 2024 Oct 04;13:RP94849. doi: 10.7554/eLife.94849 PMID: 38168435 Free PMC article. Updated. Preprint.
-
Discovery of a new pyrimidine synthesis inhibitor eradicating glioblastoma-initiating cells.Neuro Oncol. 2020 Feb 20;22(2):229-239. doi: 10.1093/neuonc/noz170. Neuro Oncol. 2020. PMID: 31499527 Free PMC article.
-
H3K4me3 remodeling induced acquired resistance through O-GlcNAc transferase.Drug Resist Updat. 2023 Nov;71:100993. doi: 10.1016/j.drup.2023.100993. Epub 2023 Aug 10. Drug Resist Updat. 2023. PMID: 37639774 Free PMC article.
-
O-linked N-acetylglucosamine transferase enhances secretory clusterin expression via liver X receptors and sterol response element binding protein regulation in cervical cancer.Oncotarget. 2017 Dec 21;9(4):4625-4636. doi: 10.18632/oncotarget.23588. eCollection 2018 Jan 12. Oncotarget. 2017. PMID: 29435130 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

