Ferrous iron is a significant component of bioavailable iron in cystic fibrosis airways
- PMID: 23963183
- PMCID: PMC3753050
- DOI: 10.1128/mBio.00557-13
Ferrous iron is a significant component of bioavailable iron in cystic fibrosis airways
Abstract
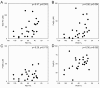
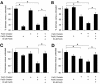
ABSTRACT Chronic, biofilm-like infections by the opportunistic pathogen Pseudomonas aeruginosa are a major cause of mortality in cystic fibrosis (CF) patients. While much is known about P. aeruginosa from laboratory studies, far less is understood about what it experiences in vivo. Iron is an important environmental parameter thought to play a central role in the development and maintenance of P. aeruginosa infections, for both anabolic and signaling purposes. Previous studies have focused on ferric iron [Fe(III)] as a target for antimicrobial therapies; however, here we show that ferrous iron [Fe(II)] is abundant in the CF lung (-39 µM on average for severely sick patients) and significantly correlates with disease severity (ρ = -0.56, P = 0.004), whereas ferric iron does not (ρ = -0.28, P = 0.179). Expression of the P. aeruginosa genes bqsRS, whose transcription is upregulated in response to Fe(II), was high in the majority of patients tested, suggesting that increased Fe(II) is bioavailable to the infectious bacterial population. Because limiting Fe(III) acquisition inhibits biofilm formation by P. aeruginosa in various oxic in vitro systems, we also tested whether interfering with Fe(II) acquisition would improve biofilm control under anoxic conditions; concurrent sequestration of both iron oxidation states resulted in a 58% reduction in biofilm accumulation and 28% increase in biofilm dissolution, a significant improvement over Fe(III) chelation treatment alone. This study demonstrates that the chemistry of infected host environments coevolves with the microbial community as infections progress, which should be considered in the design of effective treatment strategies at different stages of disease.
Importance: Iron is an important environmental parameter that helps pathogens thrive in sites of infection, including those of cystic fibrosis (CF) patients. Ferric iron chelation therapy has been proposed as a novel therapeutic strategy for CF lung infections, yet until now, the iron oxidation state has not been measured in the host. In studying mucus from the infected lungs of multiple CF patients from Europe and the United States, we found that ferric and ferrous iron change in concentration and relative proportion as infections progress; over time, ferrous iron comes to dominate the iron pool. This information is relevant to the design of novel CF therapeutics and, more broadly, to developing accurate models of chronic CF infections.
Figures


Similar articles
-
The ferrous iron-responsive BqsRS two-component system activates genes that promote cationic stress tolerance.mBio. 2015 Feb 24;6(2):e02549. doi: 10.1128/mBio.02549-14. mBio. 2015. PMID: 25714721 Free PMC article.
-
A Survival Strategy for Pseudomonas aeruginosa That Uses Exopolysaccharides To Sequester and Store Iron To Stimulate Psl-Dependent Biofilm Formation.Appl Environ Microbiol. 2016 Oct 14;82(21):6403-6413. doi: 10.1128/AEM.01307-16. Print 2016 Nov 1. Appl Environ Microbiol. 2016. PMID: 27565622 Free PMC article.
-
Competition in Biofilms between Cystic Fibrosis Isolates of Pseudomonas aeruginosa Is Shaped by R-Pyocins.mBio. 2019 Jan 29;10(1):e01828-18. doi: 10.1128/mBio.01828-18. mBio. 2019. PMID: 30696740 Free PMC article.
-
Microenvironmental characteristics and physiology of biofilms in chronic infections of CF patients are strongly affected by the host immune response.APMIS. 2017 Apr;125(4):276-288. doi: 10.1111/apm.12668. APMIS. 2017. PMID: 28407427 Review.
-
Anaerobic Pseudomonas aeruginosa and other obligately anaerobic bacterial biofilms growing in the thick airway mucus of chronically infected cystic fibrosis patients: an emerging paradigm or "Old Hat"?Expert Opin Ther Targets. 2012 Sep;16(9):859-73. doi: 10.1517/14728222.2012.708025. Epub 2012 Jul 16. Expert Opin Ther Targets. 2012. PMID: 22793158 Review.
Cited by
-
Deferiprone-Gallium-Protoporphyrin Chitogel Decreases Pseudomonas aeruginosa Biofilm Infection without Impairing Wound Healing.Materials (Basel). 2024 Feb 7;17(4):793. doi: 10.3390/ma17040793. Materials (Basel). 2024. PMID: 38399044 Free PMC article.
-
Privatisation rescues function following loss of cooperation.Elife. 2018 Dec 18;7:e38594. doi: 10.7554/eLife.38594. Elife. 2018. PMID: 30558711 Free PMC article.
-
Cystic Fibrosis-Associated Stenotrophomonas maltophilia Strain-Specific Adaptations and Responses to pH.J Bacteriol. 2019 Mar 13;201(7):e00478-18. doi: 10.1128/JB.00478-18. Print 2019 Apr 1. J Bacteriol. 2019. PMID: 30642989 Free PMC article.
-
Reaction kinetics for the biocatalytic conversion of phenazine-1-carboxylic acid to 2-hydroxyphenazine.PLoS One. 2014 Jun 6;9(6):e98537. doi: 10.1371/journal.pone.0098537. eCollection 2014. PLoS One. 2014. PMID: 24905009 Free PMC article.
-
Predicting the impact of promoter variability on regulatory outputs.Sci Rep. 2015 Dec 17;5:18238. doi: 10.1038/srep18238. Sci Rep. 2015. PMID: 26675057 Free PMC article.
References
-
- Rajan S, Saiman L. 2002. Pulmonary infections in patients with cystic fibrosis. Semin. Respir. Infect. 17:47–56 - PubMed
-
- Bjarnsholt T, Jensen PO, Fiandaca MJ, Pedersen J, Hansen CR, Andersen CB, Pressler T, Givskov M, Hoiby N. 2009. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr. Pulmonol. 44:547–558 - PubMed
-
- Worlitzsch D, Tarran R, Ulrich M, Schwab U, Cekici A, Meyer KC, Birrer P, Bellon G, Berger J, Weiss T, Botzenhart K, Yankaskas JR, Randell S, Boucher RC, Döring G. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Invest. 109:317–325 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
