Antidepressant-like properties of novel HDAC6-selective inhibitors with improved brain bioavailability
- PMID: 23954848
- PMCID: PMC3870780
- DOI: 10.1038/npp.2013.207
Antidepressant-like properties of novel HDAC6-selective inhibitors with improved brain bioavailability
Abstract
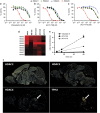
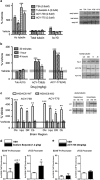
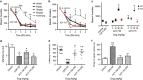
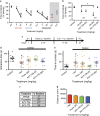
HDAC inhibitors have been reported to produce antidepressant and pro-cognitive effects in animal models, however, poor brain bioavailability or lack of isoform selectivity of current probes has limited our understanding of their mode of action. We report the characterization of novel pyrimidine hydroxyl amide small molecule inhibitors of HDAC6, brain bioavailable upon systemic administration. We show that two compounds in this family, ACY-738 and ACY-775, inhibit HDAC6 with low nanomolar potency and a selectivity of 60- to 1500-fold over class I HDACs. In contrast to tubastatin A, a reference HDAC6 inhibitor with similar potency and peripheral activity, but more limited brain bioavailability, ACY-738 and ACY-775 induce dramatic increases in α-tubulin acetylation in brain and stimulate mouse exploratory behaviors in novel, but not familiar environments. Interestingly, despite a lack of detectable effect on histone acetylation, we show that ACY-738 and ACY-775 share the antidepressant-like properties of other HDAC inhibitors, such as SAHA and MS-275, in the tail suspension test and social defeat paradigm. These effects of ACY-738 and ACY-775 are directly attributable to the inhibition of HDAC6 expressed centrally, as they are fully abrogated in mice with a neural-specific loss of function of HDAC6. Furthermore, administered in combination, a behaviorally inactive dose of ACY-738 markedly potentiates the anti-immobility activity of a subactive dose of the selective serotonin reuptake inhibitor citalopram. Our results validate new isoform-selective probes for in vivo pharmacological studies of HDAC6 in the CNS and reinforce the viability of this HDAC isoform as a potential target for antidepressant development.
Figures
Similar articles
-
Tubastatin A/ACY-1215 improves cognition in Alzheimer's disease transgenic mice.J Alzheimers Dis. 2014;41(4):1193-1205. doi: 10.3233/JAD-140066. J Alzheimers Dis. 2014. PMID: 24844691
-
Discovery of the First Irreversible HDAC6 Isoform Selective Inhibitor with Potent Anti-Multiple Myeloma Activity.J Med Chem. 2023 Jul 27;66(14):10080-10091. doi: 10.1021/acs.jmedchem.3c00977. Epub 2023 Jul 18. J Med Chem. 2023. PMID: 37463038
-
HDAC6 selective inhibition of melanoma patient T-cells augments anti-tumor characteristics.J Immunother Cancer. 2019 Feb 6;7(1):33. doi: 10.1186/s40425-019-0517-0. J Immunother Cancer. 2019. PMID: 30728070 Free PMC article.
-
A Review of Progress in Histone Deacetylase 6 Inhibitors Research: Structural Specificity and Functional Diversity.J Med Chem. 2021 Feb 11;64(3):1362-1391. doi: 10.1021/acs.jmedchem.0c01782. Epub 2021 Feb 1. J Med Chem. 2021. PMID: 33523672 Review.
-
The Therapeutic Strategy of HDAC6 Inhibitors in Lymphoproliferative Disease.Int J Mol Sci. 2018 Aug 9;19(8):2337. doi: 10.3390/ijms19082337. Int J Mol Sci. 2018. PMID: 30096875 Free PMC article. Review.
Cited by
-
The HDAC6 Inhibitor Trichostatin A Acetylates Microtubules and Protects Axons From Excitotoxin-Induced Degeneration in a Compartmented Culture Model.Front Neurosci. 2018 Nov 29;12:872. doi: 10.3389/fnins.2018.00872. eCollection 2018. Front Neurosci. 2018. PMID: 30555293 Free PMC article.
-
Design, Synthesis, Bioactivity Evaluation, Crystal Structures, and In Silico Studies of New α-Amino Amide Derivatives as Potential Histone Deacetylase 6 Inhibitors.Molecules. 2022 May 22;27(10):3335. doi: 10.3390/molecules27103335. Molecules. 2022. PMID: 35630812 Free PMC article.
-
HDAC6-selective inhibitors decrease nerve-injury and inflammation-associated mechanical hypersensitivity in mice.Psychopharmacology (Berl). 2020 Jul;237(7):2139-2149. doi: 10.1007/s00213-020-05525-9. Epub 2020 May 9. Psychopharmacology (Berl). 2020. PMID: 32388618 Free PMC article.
-
The Selective HDAC6 Inhibitor ACY-738 Impacts Memory and Disease Regulation in an Animal Model of Multiple Sclerosis.Front Neurol. 2019 Jun 28;10:519. doi: 10.3389/fneur.2019.00519. eCollection 2019. Front Neurol. 2019. PMID: 31316445 Free PMC article.
-
Epigenetic pathway targets for the treatment of disease: accelerating progress in the development of pharmacological tools: IUPHAR Review 11.Br J Pharmacol. 2014 Nov;171(22):4981-5010. doi: 10.1111/bph.12848. Br J Pharmacol. 2014. PMID: 25060293 Free PMC article. Review.
References
-
- Albelda N, Joel D. Animal models of obsessive-compulsive disorder: exploring pharmacology and neural substrates. Neurosci Biobehav Rev. 2012;36:47–63. - PubMed
-
- Benner L, Kalin JH, Kozikowski AP, Gordon MN, Morgan D, SM-L B.2012Selective HDAC6 inhibition decreases tau levels and improves spatial memory deficits in the rtg4510 mouse model Society Neurosc 2012Program No. 150.04 http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=804c6b94-1e77... .
-
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. - PubMed
-
- Bertrand P. Inside HDAC with HDAC inhibitors. Eur J Med Chem. 2010;45:2095–2116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

