Dual-energy precursor and nuclear erythroid-related factor 2 activator treatment additively improve redox glutathione levels and neuron survival in aging and Alzheimer mouse neurons upstream of reactive oxygen species
- PMID: 23954169
- PMCID: PMC6099064
- DOI: 10.1016/j.neurobiolaging.2013.06.023
Dual-energy precursor and nuclear erythroid-related factor 2 activator treatment additively improve redox glutathione levels and neuron survival in aging and Alzheimer mouse neurons upstream of reactive oxygen species
Abstract
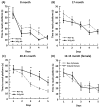
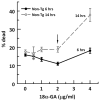
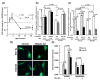
To determine whether glutathione (GSH) loss or increased reactive oxygen species (ROS) are more important to neuron loss, aging, and Alzheimer's disease (AD), we stressed or boosted GSH levels in neurons isolated from aging 3xTg-AD neurons compared with those from age-matched nontransgenic (non-Tg) neurons. Here, using titrating with buthionine sulfoximine, an inhibitor of γ-glutamyl cysteine synthetase (GCL), we observed that GSH depletion increased neuronal death of 3xTg-AD cultured neurons at increasing rates across the age span, whereas non-Tg neurons were resistant to GSH depletion until old age. Remarkably, the rate of neuron loss with ROS did not increase in old age and was the same for both genotypes, which indicates that cognitive deficits in the AD model were not caused by ROS. Therefore, we targeted for neuroprotection activation of the redox sensitive transcription factor, nuclear erythroid-related factor 2 (Nrf2) by 18 alpha glycyrrhetinic acid to stimulate GSH synthesis through GCL. This balanced stimulation of a number of redox enzymes restored the lower levels of Nrf2 and GCL seen in 3xTg-AD neurons compared with those of non-Tg neurons and promoted translocation of Nrf2 to the nucleus. By combining the Nrf2 activator together with the NADH precursor, nicotinamide, we increased neuron survival against amyloid beta stress in an additive manner. These stress tests and neuroprotective treatments suggest that the redox environment is more important for neuron survival than ROS. The dual neuroprotective treatment with nicotinamide and an Nrf2 inducer indicates that these age-related and AD-related changes are reversible.
Keywords: 3xTg-AD; Aging; Glutathione; Neurodegeneration; Neuroprotection; Nrf2; ROS; Stress.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no conflicts of interest.
Figures




Similar articles
-
External cys/cySS redox state modification controls the intracellular redox state and neurodegeneration via Akt in aging and Alzheimer's disease mouse model neurons.J Alzheimers Dis. 2014;42(1):313-24. doi: 10.3233/JAD-132756. J Alzheimers Dis. 2014. PMID: 24844688 Free PMC article.
-
A reversible early oxidized redox state that precedes macromolecular ROS damage in aging nontransgenic and 3xTg-AD mouse neurons.J Neurosci. 2012 Apr 25;32(17):5821-32. doi: 10.1523/JNEUROSCI.6192-11.2012. J Neurosci. 2012. PMID: 22539844 Free PMC article.
-
Relative importance of redox buffers GSH and NAD(P)H in age-related neurodegeneration and Alzheimer disease-like mouse neurons.Aging Cell. 2014 Aug;13(4):631-40. doi: 10.1111/acel.12216. Epub 2014 Mar 21. Aging Cell. 2014. PMID: 24655393 Free PMC article.
-
Therapeutic Approaches to Alzheimer's Disease Through Modulation of NRF2.Neuromolecular Med. 2019 Mar;21(1):1-11. doi: 10.1007/s12017-018-08523-5. Epub 2019 Jan 7. Neuromolecular Med. 2019. PMID: 30617737 Review.
-
The Role of the Transcription Factor Nrf2 in Alzheimer's Disease: Therapeutic Opportunities.Biomolecules. 2023 Mar 17;13(3):549. doi: 10.3390/biom13030549. Biomolecules. 2023. PMID: 36979483 Free PMC article. Review.
Cited by
-
Are Astrocytes the Predominant Cell Type for Activation of Nrf2 in Aging and Neurodegeneration?Antioxidants (Basel). 2017 Aug 18;6(3):65. doi: 10.3390/antiox6030065. Antioxidants (Basel). 2017. PMID: 28820437 Free PMC article. Review.
-
Brain Energy Deficit as a Source of Oxidative Stress in Migraine: A Molecular Basis for Migraine Susceptibility.Neurochem Res. 2021 Aug;46(8):1913-1932. doi: 10.1007/s11064-021-03335-9. Epub 2021 Apr 30. Neurochem Res. 2021. PMID: 33939061 Review.
-
External cys/cySS redox state modification controls the intracellular redox state and neurodegeneration via Akt in aging and Alzheimer's disease mouse model neurons.J Alzheimers Dis. 2014;42(1):313-24. doi: 10.3233/JAD-132756. J Alzheimers Dis. 2014. PMID: 24844688 Free PMC article.
-
Nrf2 regulates ROS production by mitochondria and NADPH oxidase.Biochim Biophys Acta. 2015 Apr;1850(4):794-801. doi: 10.1016/j.bbagen.2014.11.021. Epub 2014 Dec 5. Biochim Biophys Acta. 2015. PMID: 25484314 Free PMC article.
-
Mini-GAGR, an intranasally applied polysaccharide, activates the neuronal Nrf2-mediated antioxidant defense system.J Biol Chem. 2018 Nov 23;293(47):18242-18269. doi: 10.1074/jbc.RA117.001245. Epub 2018 Oct 3. J Biol Chem. 2018. PMID: 30282635 Free PMC article.
References
-
- Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer’s disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–88. - PubMed
-
- Blanc EM, Bruce-Keller AJ, Mattson MP. Astrocytic gap junctional communication decreases neuronal vulnerability to oxidative stress-induced disruption of Ca 2+ homeostasis and cell death. J Neurochem. 1998;70:958–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

