Highly potent HIV-1 protease inhibitors with novel tricyclic P2 ligands: design, synthesis, and protein-ligand X-ray studies
- PMID: 23947685
- PMCID: PMC3800042
- DOI: 10.1021/jm400768f
Highly potent HIV-1 protease inhibitors with novel tricyclic P2 ligands: design, synthesis, and protein-ligand X-ray studies
Abstract
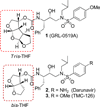
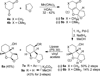
The design, synthesis, and biological evaluation of a series of HIV-1 protease inhibitors incorporating stereochemically defined fused tricyclic P2 ligands are described. Various substituent effects were investigated to maximize the ligand-binding site interactions in the protease active site. Inhibitors 16a and 16f showed excellent enzyme inhibitory and antiviral activity, although the incorporation of sulfone functionality resulted in a decrease in potency. Both inhibitors 16a and 16f maintained activity against a panel of multidrug resistant HIV-1 variants. A high-resolution X-ray crystal structure of 16a-bound HIV-1 protease revealed important molecular insights into the ligand-binding site interactions, which may account for the inhibitor's potent antiviral activity and excellent resistance profiles.
Figures
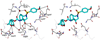
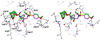
Similar articles
-
Design and Synthesis of Highly Potent HIV-1 Protease Inhibitors Containing Tricyclic Fused Ring Systems as Novel P2 Ligands: Structure-Activity Studies, Biological and X-ray Structural Analysis.J Med Chem. 2018 May 24;61(10):4561-4577. doi: 10.1021/acs.jmedchem.8b00298. Epub 2018 May 15. J Med Chem. 2018. PMID: 29763303 Free PMC article.
-
Structure-Based Design of Highly Potent HIV-1 Protease Inhibitors Containing New Tricyclic Ring P2-Ligands: Design, Synthesis, Biological, and X-ray Structural Studies.J Med Chem. 2020 May 14;63(9):4867-4879. doi: 10.1021/acs.jmedchem.0c00202. Epub 2020 Apr 29. J Med Chem. 2020. PMID: 32348139 Free PMC article.
-
Design and synthesis of potent HIV-1 protease inhibitors incorporating hexahydrofuropyranol-derived high affinity P(2) ligands: structure-activity studies and biological evaluation.J Med Chem. 2011 Jan 27;54(2):622-34. doi: 10.1021/jm1012787. Epub 2010 Dec 31. J Med Chem. 2011. PMID: 21194227 Free PMC article.
-
Design of HIV protease inhibitors targeting protein backbone: an effective strategy for combating drug resistance.Acc Chem Res. 2008 Jan;41(1):78-86. doi: 10.1021/ar7001232. Epub 2007 Aug 28. Acc Chem Res. 2008. PMID: 17722874 Review.
-
Novel strategies for targeting the dimerization interface of HIV protease with cross-linked interfacial peptides.Biopolymers. 2002;66(2):126-33. doi: 10.1002/bip.10232. Biopolymers. 2002. PMID: 12325162 Review.
Cited by
-
Nature Inspired Molecular Design: Stereoselective Synthesis of Bicyclic and Polycyclic Ethers for Potent HIV-1 Protease Inhibitors.Asian J Org Chem. 2018 Aug;7(8):1448-1466. doi: 10.1002/ajoc.201800255. Epub 2018 Jun 8. Asian J Org Chem. 2018. PMID: 31595212 Free PMC article.
-
A novel tricyclic ligand-containing nonpeptidic HIV-1 protease inhibitor, GRL-0739, effectively inhibits the replication of multidrug-resistant HIV-1 variants and has a desirable central nervous system penetration property in vitro.Antimicrob Agents Chemother. 2015 May;59(5):2625-35. doi: 10.1128/AAC.04757-14. Epub 2015 Feb 17. Antimicrob Agents Chemother. 2015. PMID: 25691652 Free PMC article.
-
Structural Adaptation of Darunavir Analogues against Primary Mutations in HIV-1 Protease.ACS Infect Dis. 2019 Feb 8;5(2):316-325. doi: 10.1021/acsinfecdis.8b00336. Epub 2018 Dec 31. ACS Infect Dis. 2019. PMID: 30543749 Free PMC article.
-
Current perspectives on HIV-1 antiretroviral drug resistance.Viruses. 2014 Oct 24;6(10):4095-139. doi: 10.3390/v6104095. Viruses. 2014. PMID: 25341668 Free PMC article. Review.
-
A fission yeast cell-based system for multidrug resistant HIV-1 proteases.Cell Biosci. 2017 Jan 11;7:5. doi: 10.1186/s13578-016-0131-5. eCollection 2017. Cell Biosci. 2017. PMID: 28096973 Free PMC article.
References
-
- Conway B. HAART in Treatment--experienced Patients in the 21st Century: The Audacity of Hope. Future Virol. 2009;4:39–41.
-
- Little SJ, Holte S, Routy JP, Daar ES, Markowitz M, Collier AC, Koup RA, Mellors JW, Connick E, Conway B, Kilby M, Wang L, Whitcomb JM, Hellmann NS, Richman D. Antiretroviral-Drug Resistance among Patients Recently Infected with HIV. N. Engl. J. Med. 2002;347:385–394. - PubMed
-
- Ghosh AK, Chapsal BD, Weber IT, Mitsuya H. Design of HIV Protease Inhibitors Targeting Protein Backbone: An Effective Strategy for Combating Drug Resistance. Acc. Chem. Res. 2008;41:78–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

