Effects of pH on the production of phosphate and pyrophosphate by matrix vesicles' biomimetics
- PMID: 23942722
- PMCID: PMC3752608
- DOI: 10.1007/s00223-013-9745-3
Effects of pH on the production of phosphate and pyrophosphate by matrix vesicles' biomimetics
Abstract
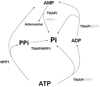
During endochondral bone formation, chondrocytes and osteoblasts synthesize and mineralize the extracellular matrix through a process that initiates within matrix vesicles (MVs) and ends with bone mineral propagation onto the collagenous scaffold. pH gradients have been identified in the growth plate of long bones, but how pH changes affect the initiation of skeletal mineralization is not known. Tissue-nonspecific alkaline phosphatase (TNAP) degrades extracellular inorganic pyrophosphate (PPi), a mineralization inhibitor produced by ectonucleotide pyrophosphatase/phosphodiesterase-1 (NPP1), while contributing Pi from ATP to initiate mineralization. TNAP and NPP1, alone or combined, were reconstituted in dipalmitoylphosphatidylcholine liposomes to mimic the microenvironment of MVs. The hydrolysis of ATP, ADP, AMP, and PPi was studied at pH 8 and 9 and compared to the data determined at pH 7.4. While catalytic efficiencies in general were higher at alkaline pH, PPi hydrolysis was maximal at pH 8 and indicated a preferential utilization of PPi over ATP at pH 8 versus 9. In addition, all proteoliposomes induced mineral formation when incubated in a synthetic cartilage lymph containing 1 mM ATP as substrate and amorphous calcium phosphate or calcium-phosphate-phosphatidylserine complexes as nucleators. Propagation of mineralization was significantly more efficient at pH 7.5 and 8 than at pH 9. Since a slight pH elevation from 7.4 to 8 promotes considerably more hydrolysis of ATP, ADP, and AMP primarily by TNAP, this small pH change facilitates mineralization, especially via upregulated PPi hydrolysis by both NPP1 and TNAP, further elevating the Pi/PPi ratio, thus enhancing bone mineralization.
Figures





Similar articles
-
Proteoliposomes harboring alkaline phosphatase and nucleotide pyrophosphatase as matrix vesicle biomimetics.J Biol Chem. 2010 Mar 5;285(10):7598-609. doi: 10.1074/jbc.M109.079830. Epub 2010 Jan 4. J Biol Chem. 2010. PMID: 20048161 Free PMC article.
-
Effect of the presence of cholesterol in the interfacial microenvironment on the modulation of the alkaline phosphatase activity during in vitro mineralization.Colloids Surf B Biointerfaces. 2017 Jul 1;155:466-476. doi: 10.1016/j.colsurfb.2017.04.051. Epub 2017 Apr 26. Colloids Surf B Biointerfaces. 2017. PMID: 28472750
-
NPP1 and TNAP hydrolyze ATP synergistically during biomineralization.Purinergic Signal. 2023 Jun;19(2):353-366. doi: 10.1007/s11302-022-09882-2. Epub 2022 Jul 23. Purinergic Signal. 2023. PMID: 35870033 Free PMC article.
-
The role of phosphatases in the initiation of skeletal mineralization.Calcif Tissue Int. 2013 Oct;93(4):299-306. doi: 10.1007/s00223-012-9672-8. Epub 2012 Nov 27. Calcif Tissue Int. 2013. PMID: 23183786 Free PMC article. Review.
-
[Regulation of bone mineralization by enzymes].Clin Calcium. 2004 Jun;14(6):23-7. Clin Calcium. 2004. PMID: 15577050 Review. Japanese.
Cited by
-
Time-dependently Appeared Microenvironmental Changes and Mechanism after Cartilage or Joint Damage and the Influences on Cartilage Regeneration.Organogenesis. 2021 Oct 2;17(3-4):85-99. doi: 10.1080/15476278.2021.1991199. Epub 2021 Nov 22. Organogenesis. 2021. PMID: 34806543 Free PMC article. Review.
-
Axial mechanical loading to ex vivo mouse long bone regulates endochondral ossification and endosteal mineralization through activation of the BMP-Smad pathway during postnatal growth.Bone Rep. 2021 May 7;15:101088. doi: 10.1016/j.bonr.2021.101088. eCollection 2021 Dec. Bone Rep. 2021. PMID: 34141832 Free PMC article.
-
Magnesium ions regulate mesenchymal stem cells population and osteogenic differentiation: A fuzzy agent-based modeling approach.Comput Struct Biotechnol J. 2021 Jul 9;19:4110-4122. doi: 10.1016/j.csbj.2021.07.005. eCollection 2021. Comput Struct Biotechnol J. 2021. PMID: 34527185 Free PMC article.
-
Shedding Light on the Role of Na,K-ATPase as a Phosphatase during Matrix-Vesicle-Mediated Mineralization.Int J Mol Sci. 2022 Dec 1;23(23):15072. doi: 10.3390/ijms232315072. Int J Mol Sci. 2022. PMID: 36499456 Free PMC article.
-
Matrix vesicles from chondrocytes and osteoblasts: Their biogenesis, properties, functions and biomimetic models.Biochim Biophys Acta Gen Subj. 2018 Mar;1862(3):532-546. doi: 10.1016/j.bbagen.2017.11.005. Epub 2017 Nov 3. Biochim Biophys Acta Gen Subj. 2018. PMID: 29108957 Free PMC article. Review.
References
-
- Boskey A. In: Dynamics of Bone and Cartilage Metabolism. Seibel, Robins, Biezikian, editors. Academic Press; 2006. pp. 201–212.
-
- Meyer JL. Can biological calcification occur in the presence of pyrophosphate? Arch Biochem Biophys. 1984;231:1–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

