Resveratrol prevents dendritic cell maturation in response to advanced glycation end products
- PMID: 23936610
- PMCID: PMC3725714
- DOI: 10.1155/2013/574029
Resveratrol prevents dendritic cell maturation in response to advanced glycation end products
Abstract
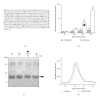
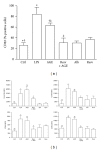
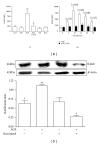
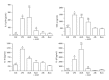
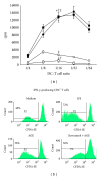
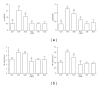
Advanced glycation end products (AGEs), generated through nonenzymatic glycosylation of proteins, lipids, and nucleic acids, accumulate in the body by age thus being considered as biomarkers of senescence. Senescence is characterized by a breakdown of immunological self-tolerance, resulting in increased reactivity to self-antigens. Previous findings suggest that AGE and its receptor RAGE may be involved in the pathogenesis of autoimmune reactions through dendritic cell (DC) activation. The aim of this study was to investigate whether resveratrol, a polyphenolic antioxidant compound with tolerogenic effects on DCs, was able to counteract the mechanisms triggered by AGE/RAGE interaction on DCs. By immunochemical and cytofluorimetric assays, we demonstrated that in vitro pretreatment of human monocyte-derived DCs with resveratrol prevents DC activation in response to glucose-treated albumin (AGE-albumin). We found that resveratrol exerts an inhibitory effect on DC surface maturation marker and RAGE up-regulation in response to AGE-albumin. It also inhibited proinflammatory cytokine expression, allostimulatory ability upregulation, mitogen-activated protein (MAP) kinases, and NF-κB activation in AGE-albumin-stimulated DCs. We suggest that resveratrol, by dismantling AGE/RAGE signaling on DCs may prevent or reduce increased reactivity to self-molecules in aging.
Figures
Similar articles
-
Advanced glycation end products of human β₂ glycoprotein I modulate the maturation and function of DCs.Blood. 2011 Jun 9;117(23):6152-61. doi: 10.1182/blood-2010-12-325514. Epub 2011 Apr 15. Blood. 2011. PMID: 21498672
-
Resveratrol prevents the impairment of advanced glycosylation end products (AGE) on macrophage lipid homeostasis by suppressing the receptor for AGE via peroxisome proliferator-activated receptor gamma activation.Int J Mol Med. 2010 May;25(5):729-34. doi: 10.3892/ijmm_00000398. Int J Mol Med. 2010. PMID: 20372816
-
Effects of glycation of the model food allergen ovalbumin on antigen uptake and presentation by human dendritic cells.Immunology. 2010 Mar;129(3):437-45. doi: 10.1111/j.1365-2567.2009.03199.x. Epub 2009 Nov 16. Immunology. 2010. PMID: 19922418 Free PMC article.
-
Advanced glycosylation end products might promote atherosclerosis through inducing the immune maturation of dendritic cells.Arterioscler Thromb Vasc Biol. 2005 Oct;25(10):2157-63. doi: 10.1161/01.ATV.0000181744.58265.63. Epub 2005 Aug 11. Arterioscler Thromb Vasc Biol. 2005. PMID: 16100036
-
Advanced glycation end products induce the expression of interleukin-6 and interleukin-8 by receptor for advanced glycation end product-mediated activation of mitogen-activated protein kinases and nuclear factor-κB in human osteoarthritis chondrocytes.Rheumatology (Oxford). 2011 May;50(5):838-51. doi: 10.1093/rheumatology/keq380. Epub 2010 Dec 20. Rheumatology (Oxford). 2011. PMID: 21172926 Free PMC article.
Cited by
-
Dietary Polyphenols, Plant Metabolites, and Allergic Disorders: A Comprehensive Review.Pharmaceuticals (Basel). 2024 May 22;17(6):670. doi: 10.3390/ph17060670. Pharmaceuticals (Basel). 2024. PMID: 38931338 Free PMC article. Review.
-
Cancer prevention and therapy through the modulation of the tumor microenvironment.Semin Cancer Biol. 2015 Dec;35 Suppl(Suppl):S199-S223. doi: 10.1016/j.semcancer.2015.02.007. Epub 2015 Apr 10. Semin Cancer Biol. 2015. PMID: 25865775 Free PMC article. Review.
-
Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition.Semin Cancer Biol. 2015 Dec;35 Suppl(Suppl):S5-S24. doi: 10.1016/j.semcancer.2015.03.005. Epub 2015 Apr 11. Semin Cancer Biol. 2015. PMID: 25869442 Free PMC article. Review.
-
The Candida albicans quorum-sensing molecule farnesol alters sphingolipid metabolism in human monocyte-derived dendritic cells.mBio. 2024 Aug 14;15(8):e0073224. doi: 10.1128/mbio.00732-24. Epub 2024 Jul 2. mBio. 2024. PMID: 38953353 Free PMC article.
-
Receptor for Advanced Glycation End Products (RAGE) in Type 1 Diabetes Pathogenesis.Curr Diab Rep. 2016 Oct;16(10):100. doi: 10.1007/s11892-016-0782-y. Curr Diab Rep. 2016. PMID: 27612847 Review.
References
-
- Thorpe SR, Baynes JW. Maillard reaction products in tissue proteins: new products and new perspectives. Amino Acids. 2003;25(3-4):275–281. - PubMed
-
- Ramasamy R, Shi FY, Schmidt AM. RAGE: therapeutic target and biomarker of the inflammatory response—the evidence mounts. Journal of Leukocyte Biology. 2009;86(3):505–512. - PubMed
-
- Shanmugam N, Kim YS, Lanting L, Natarajan R. Regulation of cyclooxygenase-2 expression in monocytes by ligation of the receptor for advanced glycation end products. The Journal of Biological Chemistry. 2003;278(37):34834–34844. - PubMed
-
- Bierhaus A, Hofmann MA, Ziegler R, Nawroth PP. AGEs and their interaction with AGE-receptors in vascular disease and diabetes mellitus. I. The AGE concept. Cardiovascular Research. 1998;37(3):586–600. - PubMed
-
- Basta G, Schmidt AM, de Caterina R. Advanced glycation end products and vascular inflammation: implications for accelerated atherosclerosis in diabetes. Cardiovascular Research. 2004;63(4):582–592. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

