Viperin regulates cellular lipid metabolism during human cytomegalovirus infection
- PMID: 23935494
- PMCID: PMC3731232
- DOI: 10.1371/journal.ppat.1003497
Viperin regulates cellular lipid metabolism during human cytomegalovirus infection
Abstract
Human cytomegalovirus (HCMV) has been shown to induce increased lipogenesis in infected cells, and this is believed to be required for proper virion envelopment. We show here that this increase is a consequence of the virus-induced redistribution of the host protein viperin to mitochondria and its capacity to interact with and block the function of the mitochondrial trifunctional protein (TFP), the enzyme that mediates fatty acid-β-oxidation. The resulting decrease in cellular ATP levels activates the enzyme AMP-activated protein kinase (AMPK), which induces expression of the glucose transporter GLUT4, resulting in increased glucose import and translocation to the nucleus of the glucose-regulated transcription factor ChREBP. This induces increased transcription of genes encoding lipogenic enzymes, increased lipid synthesis and lipid droplet accumulation, and generation of the viral envelope. Viperin-dependent lipogenesis is required for optimal production of infectious virus. We show that all of these metabolic outcomes can be replicated by direct targeting of viperin to mitochondria in the absence of HCMV infection, and that the motif responsible for Fe-S cluster binding by viperin is essential. The data indicate that viperin is the major effector underlying the ability of HCMV to regulate cellular lipid metabolism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
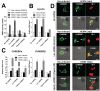
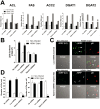
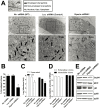
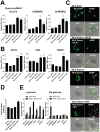
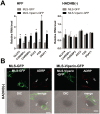
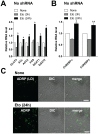
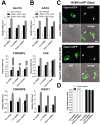
Similar articles
-
Human cytomegalovirus directly induces the antiviral protein viperin to enhance infectivity.Science. 2011 May 27;332(6033):1093-7. doi: 10.1126/science.1202007. Epub 2011 Apr 28. Science. 2011. PMID: 21527675
-
Targeting viperin to the mitochondrion inhibits the thiolase activity of the trifunctional enzyme complex.J Biol Chem. 2020 Feb 28;295(9):2839-2849. doi: 10.1074/jbc.RA119.011526. Epub 2020 Jan 24. J Biol Chem. 2020. PMID: 31980458 Free PMC article.
-
Human Cytomegalovirus pUL37x1 Is Important for Remodeling of Host Lipid Metabolism.J Virol. 2019 Oct 15;93(21):e00843-19. doi: 10.1128/JVI.00843-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31391267 Free PMC article.
-
Mechanisms of AMPK in the maintenance of ATP balance during energy metabolism.Cell Biol Int. 2018 Apr;42(4):384-392. doi: 10.1002/cbin.10915. Epub 2018 Jan 3. Cell Biol Int. 2018. PMID: 29205673 Review.
-
AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives.Acta Physiol (Oxf). 2009 May;196(1):81-98. doi: 10.1111/j.1748-1716.2009.01970.x. Epub 2009 Feb 19. Acta Physiol (Oxf). 2009. PMID: 19245656 Free PMC article. Review.
Cited by
-
Viperin Reveals Its True Function.Annu Rev Virol. 2020 Sep 29;7(1):421-446. doi: 10.1146/annurev-virology-011720-095930. Epub 2020 Jun 30. Annu Rev Virol. 2020. PMID: 32603630 Free PMC article. Review.
-
A key anti-viral protein, RSAD2/VIPERIN, restricts the release of measles virus from infected cells.Virus Res. 2019 Apr 2;263:145-150. doi: 10.1016/j.virusres.2019.01.014. Epub 2019 Jan 23. Virus Res. 2019. PMID: 30684519 Free PMC article.
-
Progression of pathology in PINK1-deficient mouse brain from splicing via ubiquitination, ER stress, and mitophagy changes to neuroinflammation.J Neuroinflammation. 2017 Aug 2;14(1):154. doi: 10.1186/s12974-017-0928-0. J Neuroinflammation. 2017. PMID: 28768533 Free PMC article.
-
A Cysteine Residue of Human Cytomegalovirus vMIA Protein Plays a Crucial Role in Viperin Trafficking to Control Viral Infectivity.J Virol. 2023 Jun 29;97(6):e0187422. doi: 10.1128/jvi.01874-22. Epub 2023 Jun 12. J Virol. 2023. PMID: 37306568 Free PMC article.
-
Identification of HNF-4α as a key transcription factor to promote ChREBP expression in response to glucose.Sci Rep. 2016 Mar 31;6:23944. doi: 10.1038/srep23944. Sci Rep. 2016. PMID: 27029511 Free PMC article.
References
-
- Britt WJ, Alford CA (1996) Cytomegalovirus. In: Fields BN, Knipe DM, Howley PM, editors. Fields Virology, Third Edition. New York: Raven Press. pp. 2493–2523.
-
- Rubin R (2002) Clinical approach to infection in the compromised host. In: Rubin R, Young LS, editor. Infection in the Organ Transplant Recipient. New York: Kluwer Academic Press. pp. 573–679.
-
- Stagno S, Britt WJ (2005) Cytomegalovirus. In: Remington JS, Klein JO, editors. Infectious Diseases of the Fetus and Newborn Infant. 6th Edition. Philadelphia: W.B. Saunders.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

